1836
Early results from a patient study aimed at testing a quantitative and synthetic brain MRI method, for abbreviated neuro protocols1Computer Science and Engineering, National Sun Yat-sen University, Kaohsiung, Taiwan, 2Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
We developed a quantitative and synthetic MRI method capable of generating the most-commonly encountered contrasts in neuro protocols, along with quantitative parameter maps, with full brain coverage and 1-mm isotropic resolution, from only about 5 min of scanning. The eventual goal is for this sequence and associated processing to play a role in abbreviated neuro protocols. Promising results were obtained in healthy volunteers and we recently embarked on a larger patient study. After a few months of delay, largely associated with the pandemic, patient recruitment has started, and preliminary results are shown here.
Introduction
Abbreviated protocols represent a way to potentially increase value in MRI 1, by reducing exam times to only a few minutes and, in the process, hopefully increase accessibility and reduce costs 2-4. The present approach aims to generate nearly all contrasts typically encountered in a basic neuro protocol, but from only a few minutes of scan time. It further aims at generating quantitative maps, such as T1 and T2 maps.The goals of the present approach are similar to those of better-known methods such as MR fingerprinting (MRF) 5,6 and SyMRI (also known as MAGiC on GE scanners) 7-9. A strength of the present method is its imaging speed, which allows superior coverage/resolution to be achieved; with MRF or SyMRI, achieving full brain coverage in ~5 min would likely involve 2D slices several-mm in thickness with several-mm gaps in-between them, while here it is achieved with (no-gap) isotropic 1-mm resolution instead. However, the present method is at an earlier stage of development, and not all contrasts may yet be generated with the desired quality.
A manuscript presenting a small volunteer study, with the present acquisition / reconstruction strategy, reached the finals of the ISMRM YIA competition last year 10. The present work aimed at improving the pulse sequence, and at performing validation in a larger study involving patients. After several delays, which were caused in large part by the pandemic, recruitment has finally recently started, with 2 patients scanned. Although our goal is to ultimately abbreviate even brain exams that include an injection of contrast agent, in the present study and as typically done in the field quantitative/synthetic MRI we are starting here with simpler non-contrast exams, as a first step.
Methods
Recruited patients provided written informed consent, with an IRB-approved protocol. A multi-pathway multi-echo (MPME) 3D scan was added to their regular clinical exam (3T Siemens Prisma, 20-channel head coil, 4 pathways, 3 TEs, TR = 15ms, α = 30°, FOV = 208×220×220 mm3, 1-mm isotropic resolution, golden-angle radial acquisition in the ky-kz plane, 102 ky-kz spokes, acceleration of 3.4 vs. a fully-sampled radial acquisition, 5min37s scan time). The BART toolbox 11 was employed as part of the image reconstruction. Compared to the prior volunteer study performed with this approach 10, patient MPME results have higher spatial resolution, greater number of echo times, and shorter scan times. These improvements were made possible through improvements to the pulse sequence, and by more-fully exploiting the achievable gradient strength and slew rate of a given scanner. The reconstructed MPME images are meant to be contrast-translated into all targeted quantitative and qualitative contrast; however, in this case, the present patient study was still too preliminary for a neural network to be successfully trained for contrast translation.Results
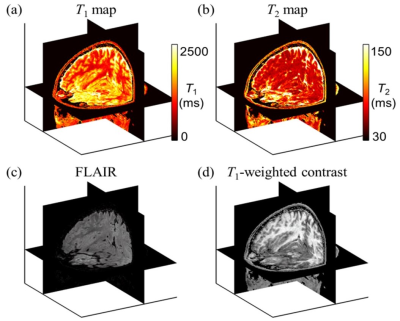
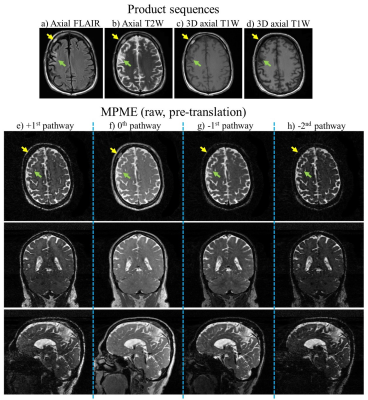
Figure 1 involves prior results, from the healthy volunteer study, to show an example of fully contrast-translated images. Using a trained neural network, the acquired raw MPME images were contrast-translated on a voxel-by-voxel basis into several different quantitative and qualitative contrasts: T1 maps, T2 maps, MPRAGE, FLAIR, T1 -weighted, T2 -weighted, and proton density [PD]-weighted.Figure 2 shows results from the present patient study, more specifically from patient #2. Images from product sequences as well as raw (non-translated) MPME images are shown. Because the number of recruited patients is still too small to train a neural network, only the native MPME contrast is shown, as opposed to contrast-translated ones.
The location of an evolving infarct is highlighted using a green arrow, and a benign hemangioma in the right frontal bone using a yellow arrow. The various image contrasts and slices shown here capture these two findings with varying degrees of conspicuity.
Discussion
MPME images are not meant to be read directly, but rather to be translated into common contrast types such as those in Fig. 2a-d, or Fig. 1. When the number of recruited patients becomes sufficient, a neural network will be trained to translate the raw images from the latest version of the MPME sequence, as shown in Fig. 2e-h, into a variety of quantitative and qualitative contrasts (T1 maps, T2 maps, MPRAGE, FLAIR, T1-weighted, T2-weighted, and proton density [PD]-weighted).Conclusion
An MPME sequence able to provide a 1-mm resolution with isotropic full brain coverage in 5min37s was developed and tested in two patients, as part of an ongoing patient study. Neural-based contrast translation will be performed once the number of recruited patients becomes large enough for training and validation purposes.Acknowledgements
Financial support from grants NIH P41EB015898 and R03EB025546 is duly acknowledged.References
1. van Beek EJR, Kuhl C, Anzai Y, et al. Value of MRI in medicine: More than just another test? J Magn Reson Imaging. 2019;49(7):e14-e25.
2. Leithner D, Moy L, Morris EA, Marino MA, Helbich TH, Pinker K. Abbreviated MRI of the Breast: Does It Provide Value? J Magn Reson Imaging. 2019;49(7):e85-e100.
3. Link TM, Patel R. The need for short MRI examinations: A musculoskeletal perspective. J Magn Reson Imaging. 2019;49(7):e49-e50.
4. Deike-Hofmann K, Koenig F, Paech D, et al. Abbreviated MRI Protocols in Breast Cancer Diagnostics. J Magn Reson Imaging. 2019;49(3):647-658.
5. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187-192.
6. Korzdorfer G, Kirsch R, Liu K, et al. Reproducibility and Repeatability of MR Fingerprinting Relaxometry in the Human Brain. Radiology. 2019;292(2):429-437.
7. Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson Med. 2008;60(2):320-329.
8. Blystad I, Warntjes JB, Smedby O, Landtblom AM, Lundberg P, Larsson EM. Synthetic MRI of the brain in a clinical setting. Acta Radiol. 2012;53(10):1158-1163.
9. Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. AJNR Am J Neuroradiol. 2017;38(6):1103-1110.
10. Cheng CC, Preiswerk F, Madore B. Multi-pathway multi-echo acquisition and neural contrast translation to generate a variety of quantitative and qualitative image contrasts. Magn Reson Med. 2020;83(6):2310-2321.
11. Uecker M, Tamir J, Lustig M. BART Toolbox. https://mrirecongithubio/bart/.
Figures