1832
Dipole inversion by recurrent inference for quantitative susceptibility mapping1Department of Radiology and Nuclear Medicine, Erasmus MC, Rotterdam, Netherlands, 2Department of Imaging Physics, TU Delft, Delft, Netherlands
Synopsis
QSM dipole inversion remains a challenge and recent machine learning approaches have not incorporated the known forward model directly. We propose using recurrent inference machines (RIM), a type of unrolled optimization technique, which are proposed for solving iterative inverse problems specifically. RIMs enable incorporating the forward dipole convolution directly in the learning process. Simulated data was used for training. The QSM reconstruction was tested on simulated data and healthy subject data acquired at 3T.
Introduction and background
Quantitative susceptibility mapping (QSM) is a promising quantitative MR technique sensitive to tissue susceptibilities. QSM can be used to depict iron deposits, small veins, calcium and myelin1,2. The dipole inversion step unfolds the biophysical effect of tissue susceptibility on the measured MR signal and is a challenge in the post-processing pipeline.The forward effect, under certain assumptions, of tissue susceptibility $$$\chi(r)$$$ to field shift $$$\delta f(r)$$$ can be written as a spatial convolution $$$\delta f=\chi\ast A$$$ or a Fourier multiplication1,3
$$\delta f(\overrightarrow{r})=\int_{r_d} \chi(\overrightarrow{r}')\frac{3cos^2(\theta )-1}{4\pi |\overrightarrow{r}'-\overrightarrow{r}|}d^3r'=iFT\big( FT(\chi(\overrightarrow{r}))\times (\frac{1}{3}-\frac{k_z}{k^2})\big) \quad[1]$$
where $$$A$$$ is the dipole kernel, $$$\overrightarrow{r}$$$ the spatial location vector, $$$\theta$$$ denotes the angle between field $$$B_0$$$ and $$$|\overrightarrow{r}’-\overrightarrow{r}|$$$, $$$r_d$$$ the spatial domain sufficiently distant from $$$r$$$, (i)FT denotes an (inverse) 3D Fourier operation, and $$$\overrightarrow{k}$$$ is the frequency vector. The dipole kernel $$$A$$$ has zero-values in both spatial and frequency domains and consequently a direct inversion from $$$\delta f $$$ to $$$\chi$$$ is infeasible.
Conventional techniques use modified kernels with near zero-values4 or iterative regularized reconstruction techniques5,6 while recent machine-learning approaches use 3D U-NETs7–10 or variational regularisers11. Recently inverse problem solving recurrent architectures12–14, and specifically Recurrent Inference Machines (RIM) have been developed15,16. RIMs perform inversion through a fixed number of inferences, and could aid machine interpretability17.
RIMs use the forward model (equation 1) and during training learn an implicit prior on the model parameters (Figure 1). The parameters at inference step $$$t$$$ are updated as $$\chi_{t+1}=\chi_t+g_{\phi}(\nabla L(\chi_t,A),\chi_t)\quad[2]$$ where the likelihood function $$$L(\chi_t,A)=\sum_{\overrightarrow{r}}(\chi_t \ast A - M)^2$$$ evaluates consistency with the input field map $$$M$$$ and $$$g_{\phi}$$$ is a Recurrent Neural Network (RNN) with the learned parameters $$$\phi$$$. The RIM is trained as
$$ \phi = \arg \min_{\phi} E_{\phi^{GT},M}\sum_t |\chi_t-\chi^{GT}|^2\quad[3]$$ where $$$\chi^{GT}$$$ denotes the ground truth susceptibility.
We developed a RIM for the purpose of dipole inversion and compare it against MEDI5.
Methods
The RIM was implemented in PyTorch with 6 inference steps using identical RNNs (kernel size 3, padding to preserve image size at each layer, 3D convolution stride 1). Training (batch size =10, ADAM optimizer, learning rate 1e-5) used 10’000 cubic samples of size 32 which served as ground truth susceptibility $$$\chi^{GT}$$$. These contained 60 randomly placed rectangles (p=2/3) and spheres (p=1/3) with random sizes and specific susceptibilities drawn from a normal distribution ($$$\mu$$$=0,$$$\sigma$$$=1), similar to DeepQSM7. Input fields $$$M$$$ were created using equation 1 without added noise. Training with 50 epochs took 15 hours on an NVIDIA RTX 2080 Ti. No validation was used. Testing was done using 1000 new samples created akin to training. For comparison MEDI5 reconstructions were made ($$$\lambda$$$=250).In-vivo data was collected from a healthy volunteer using a 3T GE Discovery MR750 with an 8 channel brain coil. A 3D multi-echo gradient-echo sequence ($$$TE_1$$$=13ms,$$$\Delta TE$$$=3.3ms, $$$TE_{16}$$$=65.8ms)18 with 1mm isotropic resolution, covering from brain-stem to above deep gray nuclei. The last 3 echoes were discarded after visual inspection. Coil images were combined using estimated sensitivities19,20. BET21 was used for masking, while frequency fields and phase unwrapped images were created using the MEDI toolbox22–24. Projection onto Dipole Fields (PDF) was used to remove background fields25. The relative difference fields (RDF) acted as the input field maps $$$M$$$ for both MEDI ($$$\lambda$$$=400, spherical radius 6.5) and RIM.
Results
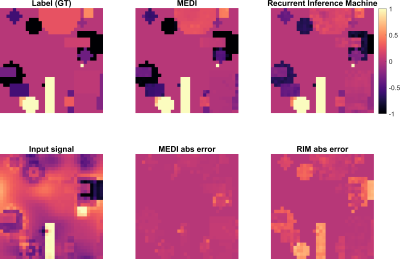
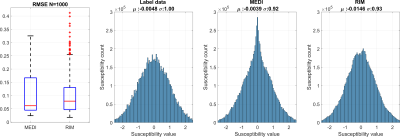
Figure 2 shows the iterative behaviour of RIM and MEDI for a testing example. Figure 3 shows simulation results between MEDI and RIM. Both approaches correctly identified shapes within the sample, however RIM over and underestimated some shapes leading to larger errors.Quantitative analysis of the simulation data (Figure 4) shows that median RMSE was 28% higher for RIM results than for MEDI. Histograms show that MEDI has less bias than the RIM, however the RIM distribution matches more closely to the original data.
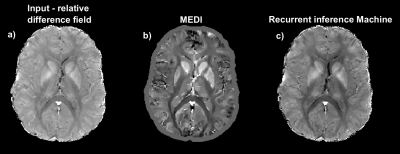
Figure 5 shows an in-vivo comparison of the input data (RDF) and the reconstructions from MEDI and RIM. MEDI successfully reconstructed high contrast in deep gray nuclei, known to have high iron content. The RIM approached darkening of myelinated regions, yet seems closer to the initial input data.
Discussion
The RIM is proposed as an alternative to 3D U-NETS for the dipole inversion problem, as they are designed to solve inverse problems based on explicit forward models. Their recurrent behaviour (Figure 2) allow for interpretability. RIM performed well on the simulation data yet was outperformed by MEDI, possibly due to estimation bias. RIM also did not perform as well as MEDI on in-vivo data. Causes could be insufficient training or unmatched training data. Future work will investigate both possibilities further, using real or simulated brain data.The choice of network architecture defines inversion behaviour, thus, including prior knowledge. The effect of the number of inference steps remains to be investigated, as is a comparison with other machine learning approaches7-11.
Conclusion
Recurrent Inference Machines (RIM) were shown to learn the dipole inversion problem from simulation data.Acknowledgements
This project was funded by Convergence for healthy and technology.
S.W. acknowledges funding from the 4TU Precision Medicine program.
References
1. Schweser F, Deistung A, Reichenbach JR. Foundations of MRI phase imaging and processing for Quantitative Susceptibility Mapping (QSM). Z. Med. Phys. 2016;26:6–34 doi: 10.1016/j.zemedi.2015.10.002.
2. Reichenbach JR, Schweser F, Serres B, Deistung A. Quantitative Susceptibility Mapping: Concepts and Applications. Clin. Neuroradiol. 2015;25:225–230 doi: 10.1007/s00062-015-0432-9.
3. de Rochefort L, Liu T, Kressler B, et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: Validation and application to brain imaging. Magn. Reson. Med. 2010;63:194–206 doi: 10.1002/mrm.22187.
4. Shmueli K, de Zwart JA, van Gelderen P, Li T-Q, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn. Reson. Med. 2009;62:1510–1522 doi: 10.1002/mrm.22135.
5. Liu J, Liu T, De Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012;59:2560–2568 doi: 10.1016/j.neuroimage.2011.08.082.
6. Li W, Wang N, Yu F, et al. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. Neuroimage 2015;108:111–122 doi: 10.1016/j.neuroimage.2014.12.043.
7. Bollmann S, Rasmussen KGB, Kristensen M, et al. DeepQSM - using deep learning to solve the dipole inversion for quantitative susceptibility mapping. Neuroimage 2019;195:373–383 doi: 10.1016/j.neuroimage.2019.03.060.
8. Yoon J, Gong E, Chatnuntawech I, et al. Quantitative susceptibility mapping using deep neural network: QSMnet. Neuroimage 2018;179:199–206 doi: 10.1016/j.neuroimage.2018.06.030.
9. Chen Y, Jakary A, Avadiappan S, Hess CP, Lupo JM. QSMGAN: Improved Quantitative Susceptibility Mapping using 3D Generative Adversarial Networks with increased receptive field. Neuroimage 2020;207:116389 doi: 10.1016/j.neuroimage.2019.116389.
10. Gao Y, Zhu X, Moffat BA, et al. xQSM: Quantitative Susceptibility Mapping with Octave Convolutional and Noise Regularized Neural Networks. 2020:1–9.
11. Polak D, Chatnuntawech I, Yoon J, et al. Nonlinear dipole inversion (NDI) enables robust quantitative susceptibility mapping (QSM). NMR Biomed. 2020;33:1–13 doi: 10.1002/nbm.4271.
12. Diamond S, Sitzmann V, Heide F, Wetzstein G. Unrolled Optimization with Deep Priors. arXiv 2017:1–11.
13. Hosseini SAH, Yaman B, Moeller S, Hong M, Akçakaya M. Dense Recurrent Neural Networks for Accelerated MRI: History-Cognizant Unrolling of Optimization Algorithms. IEEE J. Sel. Top. Signal Process. 2019;14:1280–1291 doi: 10.1109/JSTSP.2020.3003170.
14. Knoll F, Hammernik K, Kobler E, Pock T, Recht MP, Sodickson DK. Assessment of the generalization of learned image reconstruction and the potential for transfer learning. Magn. Reson. Med. 2019;81:116–128 doi: 10.1002/mrm.27355.
15. Putzky P, Welling M. Recurrent Inference Machines for Solving Inverse Problems. 2017.
16. Lønning K, Putzky P, Sonke J-J, Reneman L, Caan MWA, Welling M. Recurrent inference machines for reconstructing heterogeneous MRI data. Med. Image Anal. 2019;53:64–78 doi: 10.1016/j.media.2019.01.005.
17. Monga V, Li Y, Eldar YC. Algorithm Unrolling: Interpretable, Efficient Deep Learning for Signal and Image Processing. arXiv 2019.
18. Mazzucchi S, Frosini D, Costagli M, et al. Quantitative susceptibility mapping in atypical Parkinsonisms. NeuroImage Clin. 2019;24:101999 doi: 10.1016/j.nicl.2019.101999.
19. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn. Reson. Med. 2000;43:682–690 doi: 10.1002/(SICI)1522-2594(200005)43:5<682::AID-MRM10>3.0.CO;2-G.
20. Sun H. QSM software. https://github.com/sunhongfu/QSM.
21. Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155 doi: 10.1002/hbm.10062.
22. Kressler B, de Rochefort L, Tian Liu, Spincemaille P, Quan Jiang, Yi Wang. Nonlinear Regularization for Per Voxel Estimation of Magnetic Susceptibility Distributions From MRI Field Maps. IEEE Trans. Med. Imaging 2010;29:273–281 doi: 10.1109/TMI.2009.2023787.
23. de Rochefort L, Brown R, Prince MR, Wang Y. Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn. Reson. Med. 2008;60:1003–1009 doi: 10.1002/mrm.21710.
24. Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn. Reson. Med. 2013;69:467–476 doi: 10.1002/mrm.24272.
25. Liu T, Khalidov I, de Rochefort L, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24:1129–1136 doi: 10.1002/nbm.1670.
Figures