1823
In vivo hijacking local activated macrophages for targeted theranostics of epilepsy with nanomedicines1Fujian Medical University Union Hosptial, Fuzhou, China, 2Fudan University Huashan Hospital, Shanghai, China, 3Fujian Cancer Hospital, Fuzhou, China, 4GE Healthcare, Beijing, China
Synopsis
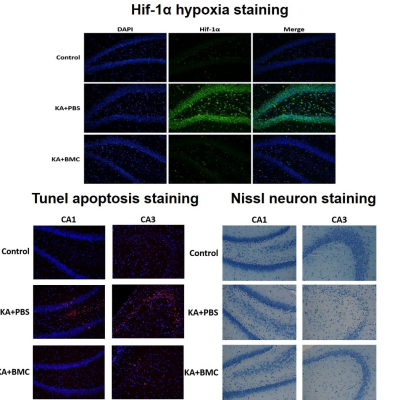
Epilepsy is closely linked to inflammation, oxidative stress, and hypoxia. The difficulty of accurate localization and targeted drug delivery to the lesion hinders the effective treatment. The local activated inflammatory cells offer a new opportunity for drug delivery to the lesion. Here, CD163 positive macrophages were first utilized as cell carriers after being hijacked by targeted albumin nanoparticles, which effectively delivered nanoparticles to the epileptic sites. Thus, accumulative nanoparticles enabled the visualization of the epileptogenic lesion though microenvironment-responsive MR-T1W imaging. Moreover, these manganese-based nanomaterials played a crucial role in protecting neurons from cell apoptosis mediated by oxidative stress and hypoxia.
Purpose
Correct localization of the epileptogenic region can improve surgical outcome in patients of epilepsy (1). Neuroinflammation, hypoxia, and reactive oxygen species (ROS) is closely linked to epilepsy and causes cell apoptosis of neurons (2, 3). In the present study, CD163+ macrophages were explored as cell carriers after selectively carrying targeted nanoparticles (NPs), which effectively infiltrated the epileptogenic region, enabled microenvironment-responsive MR-T1W imaging and neuroprotection of epileptic foci.Methods
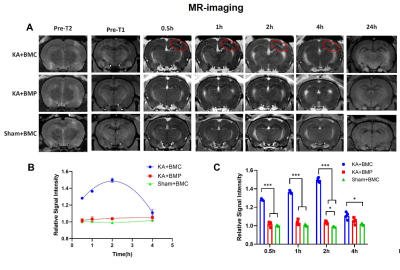
In this system, bovine serum albumin (BSA) was used as the nanocarriers to induce the formation of MnO2 nanocrystals through biomineralization, obtaining BSA-MnO2 (BM) NPs. Afterward, the CD163 peptide was covalently anchored on the BM NPs to construct CD163 peptide-modified BM NPs (BMC NPs). CD163-targeted albumin manganese dioxide (BMC) nanoparticles were first synthesized. Subsequently, a seizure rat model was established by the stereological injection of kainic acid (KA). After that, the imaging, ROS scavenging, and oxygen production ability of the nanoparticles were investigated.Results
The imaging and histopathology in combination with the molecular biology findings showed that BMC NPs can be harnessed to accurately localize the epileptogenic region with high sensitivity and specificity. Moreover, BMC NPs can synergistically scavenge ROS and generate O2 for the protection of neurons in the brain.Discussion and conclusion
This study not only demonstrated the great potential of BMC nanoparticles for microenvironment-responsive MR-T1W imaging of the epileptogenic region but also provided a new therapeutic strategy to overcoming oxidative stress and hypoxia environment of epileptic foci. Also, the present study provides an intriguing approach for targeted theranostics of epilepsy and other hypoxia-associated inflammatory diseases such as ischemic stroke.Acknowledgements