1820
MR detection of gas microbubbles via hyperCEST: a path toward a dual modality contrast agent
Christian T McHugh1, Phillip G Durham2, Michele Kelley1, Paul A Dayton3, and Rosa T Branca1
1Physics & Astronomy, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Pharmacoengineering and Molecular Pharmaceutics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
1Physics & Astronomy, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Pharmacoengineering and Molecular Pharmaceutics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Gas microbubbles are capable of providing both ultrasound and MRI image contrast, but at differing concentration levels. In order for gas microbubbles to be detected by MRI at the clinical ultrasound dose, a more sensitive detection scheme must be employed. Here we demonstrate that hyperCEST is capable of detecting gas microbubbles at clinically relevant US concentrations, thus enabling their use as a dual modality contrast agent.
Introduction
Microbubbles (MBs) are micron-sized spheres composed of a lipid shell and a heavy-gas core. MBs are typically used as an ultrasound (US) contrast agent. When in solution, the susceptibility gradients generated at the many MBs air-gas interfaces could be leveraged to produce spin dephasing and T2* contrast in MR images. In reality, even at high magnetic field strengths, MB-induced T2* contrast is small; MR detection of MBs often requires concentrations that are orders of magnitude higher than what is used for US studies1, or the addition of iron oxides to their lipid shells2. The scope of this work was to assess if MR sensitivity to gas MBs at the fM in-blood concentrations used in clinical US studies could be improved by using hyperCEST, an NMR technique that is capable of detecting extremely dilute hosts that can freely exchange hyperpolarized (HP) 129Xe with the solvent.Methods
Quantitative hyperCEST (qHyperCEST)3 with the Full HyperCEST (FHC) solution4 was used to simulate the effects of saturation of gas-phase 129Xe, which freely diffuses into and out of the MB core, on the 129Xe solvent signal. Simulations were performed for MBs with different diameters and concentrations, and for different saturation pulse strengths.Small-sized (0.9 ± 0.4 μm) and medium-sized (2 ± 1 μm) perfluorocarbon MBs were prepared in-house as previously described5. The size distributions and concentrations were determined via single particle optical sizing (Accusizer 780AD, Particle Sizing Systems, Port Richey, FL, USA). Large-sized (3.2 ± 0.7 μm), commercially-available MBs were purchased directly from Advanced Microbubbles Inc (Newark, CA, USA). Each sample was diluted to 10s-100s fM to reflect standard doses used for contrast US (10 μL/kg at 1010 MB per mL for humans, and 10x greater for rodents).
All in vitro spectroscopic studies were made on a high resolution 500 MHz NMR spectrometer (Varian NMR Systems, Palo Alto, California, USA). Before NMR signal acquisition, MB samples were equilibrated to 37 °C to replicate in vivo conditions. Fresh HP 129Xe was periodically bubbled directly into the sample, followed by a presaturation pulse (B1), a 90° continuous wave excitation pulse, and signal acquisition. The resulting Z-spectra were fit according to the FHC solution4, and qHyperCEST was used to characterize the exchange dynamics.
Results
Figure 1 shows simulation results. As expected, the hyperCEST contrast increases with gas-volume concentration as well as with B1 strength. In addition, an optimal MB size exists that provides an exchange rate that is neither too fast (where the occupation time of 129Xe inside the MB is too short to be appreciably saturated) nor too slow (where the amount of saturated 129Xe exchanging into solution is insufficient on the timescale of an experiment). Figure 2 shows experimental Z-spectra for different MB samples. Experimental data confirm the contrast behavior predicted by the simulations. Exceptional gas turnover rates provided substantial contrast even at low B1 strengths. Efficacy diminishes once the MB diameter exceeds 2 μm. Most importantly, MBs show an hyperCEST contrast at concentrations as low as 10s of fM, which is at the clinical in-blood concentration used in humans.Discussion
HyperCEST agents capable of accommodating many 129Xe atoms at a time are efficient, and are therefore detectable at concentrations much lower than carriers that maintain a 1:1 stoichiometry. Compared to perfluorocarbon nanoemulsions6 and gas vesicles7,8, MBs are kinetically and magnetically superior; they can be detected at a much lower concentrations, while their narrower gas-phase peak leads to more efficient saturation. Interestingly, while the hyperCEST effect is expected to increase with gas-volume concentration, increasing MB diameter to provide more volume comes at the cost of a reduced exchange rate, effectively requiring MB size optimization.Conclusion
MBs are capable of producing a substantial hyperCEST contrast at clinically relevant concentrations. Further, the narrower gas-phase peak characteristic of spherical gas MBs leads to more efficient saturation, lessening concerns about SAR limitations for in vivo translation. As such, hyperCEST detection of gas MBs is expected to enable the use of MBs as a dual modality contrast agent for combined US and MRI applications.Acknowledgements
No acknowledgement found.References
- Cheung JS, Chow AM, Guo H, Wu EX. Microbubbles as a novel contrast agent for brain MRI. Neuroimage. 2009;46(3):658-664. doi:10.1016/j.neuroimage.2009.02.037
- Yang F, Li Y, Chen Z, Zhang Y, Wu J, Gu N. Superparamagnetic iron oxide nanoparticle-embedded encapsulated microbubbles as dual contrast agents of magnetic resonance and ultrasound imaging. Biomaterials. 2009;30(23-24):3882-3890. doi:10.1016/j.biomaterials.2009.03.051
- Kunth M, Witte C, Schröder L. Quantitative chemical exchange saturation transfer with hyperpolarized nuclei (qHyper-CEST): Sensing xenon-host exchange dynamics and binding affinities by NMR. J Chem Phys. 2014. doi:10.1063/1.4901429
- Zaiss M, Schnurr M, Bachert P. Analytical solution for the depolarization of hyperpolarized nuclei by chemical exchange saturation transfer between free and encapsulated xenon (HyperCEST). J Chem Phys. 2012;136(14). doi:10.1063/1.3701178
- Fix SM, Nyankima AG, McSweeney MD, Tsuruta JK, Lai SK, Dayton PA. Accelerated Clearance of Ultrasound Contrast Agents Containing Polyethylene Glycol is Associated with the Generation of Anti-Polyethylene Glycol Antibodies. Ultrasound Med Biol. 2018;44(6):1266-1280. doi:10.1016/j.ultrasmedbio.2018.02.006
- Stevens TK, Ramirez RM, Pines A. Nanoemulsion contrast agents with sub-picomolar sensitivity for xenon NMR. J Am Chem Soc. 2013;135(26):9576-9579. doi:10.1021/ja402885q
- Shapiro MG, Ramirez RM, Sperling LJ, et al. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nat Chem. 2014;6(7):629-634. doi:10.1038/nchem.1934
- Kunth M, Lu GJ, Witte C, Shapiro MG, Schröder L. Protein Nanostructures Produce Self-Adjusting Hyperpolarized Magnetic Resonance Imaging Contrast through Physical Gas Partitioning. ACS Nano. 2018;12(11):10939-10948. doi:10.1021/acsnano.8b04222
Figures
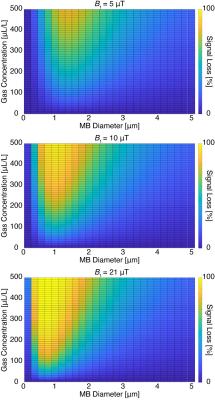
Figure 1. Simulations of MBs hyperCEST contrast. MB samples are characterized by the gas-volume concentration (μL/L) and average diameter (μm). These characteristics are directly related to the exchange rate and participation rate of 129Xe. Using the FHC solution, signal loss at different B1 strengths is shown. Signal loss generally increases with B1 and gas-volume concentration. The MB size must be optimized, and MBs lose efficiency as hyperCEST agents at sizes in excess of 2 μm.
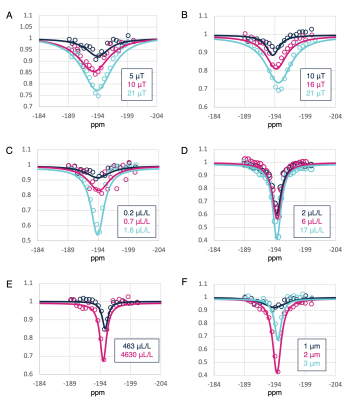
Figure 2. Zoom-in of experimental Z-spectra around the gas-phase peak. Z-spectra for (A, 340 fM) small and (B, 40 fM) medium MBs at different B1 strengths. Z spectra obtained with a constant B1 (5 μT) at different gas-volume concentrations with (C) small, (D) medium, and (E) large MBs. (F) Z-spectra of MBs with different sizes at a constant concentration of 400 fM. Signal loss increases with B1 as well as with gas-volume concentration. HyperCEST efficiency is maximized for MBs with a 2 μm diameter.