1816
Single Loop Tri-frequency Surface Coil Design for 1H MRI and Interleaved Dynamic 2H and 17O MRS Applications at Ultrahigh Field of 16.4T1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
To capture anatomical information and dynamic changes of different metabolic activities in the brain or other organs using a singular RF coil, we developed a loop RF surface coil design with a wide tune-match isolation and active tune circuitry for proton ( 1H) MRI, and interleaved deuterium ( 2H) and oxygen ( 17O) MRS measurements at 16.4T. We obtained static 1H MRI and performed interleaved dynamic 2H and 17O measurements in phantom studies. Results showed proficient proton imaging and quasi-stable 2H and 17O signals with rapid acquisition, indicating the potential of the design for robust high temporal resolution metabolic and anatomical imaging applications.
Introduction
Ultrahigh field (UHF) metabolic imaging is important for understanding physiologic mechanisms in animal and human applications1-3. It often relies on multi-nuclei measurements for anatomic and metabolic information; therefore, the RF probe generally includes multiple coils tuned to a specific frequency or a singular coil that can be manually tuned to multiple frequencies3-10. However, these approaches alone are challenging for interleaved or simultaneous scanning to capture multi-nuclei metabolic information and dynamics. Herein we report a new singular RF coil design11,12 with active tuning/matching for proton ( 1H) MRI, and interleaved deuterium ( 2H) and oxygen ( 17O) MRS measurements at 16.4T.Methods
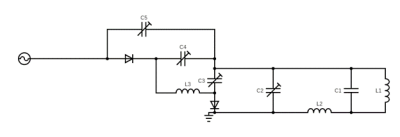
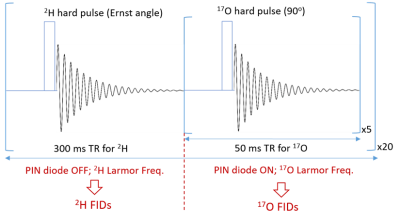
To achieve a wide range tuning capability (from 698MHz for 1H down to 94MHz for 17O at 16.4T), a traditional loop coil with distributed capacitance was reworked as shown in Figure 1. It has been shown that one can achieve wide-range tuning through the addition of an isolating inductor that separates two primary resonant circuits11,12. For this application, the isolating inductor was used to achieve robust tuning and matching between 1H (698MHz) and 2H (107Mz) frequencies. For interleaved metabolic measurements, the coil must actively switch between the 2H and 17O frequencies. Using PIN diodes and feeding the coaxial line with RF and DC powers, one can bias diodes and run RF through parallel capacitors to add capacitance in tuning and matching capacitors to drop the resonant frequency from 107MHz ( 2H) to 94MHz ( 17O), thereby providing an active tuning between 2H and 17O measurements. Tuning and matching of 1H and 2H were performed manually. The circuit was modeled in Keysight’s ADS RF simulation software selecting component values that minimized reflection coefficients at the three target resonant frequencies. The circuit was built to specification, traps were added to minimize coaxial line distortion and the loaded circuit was validated on a network analyzer measuring reflection coefficients.The new coil was tested at 16.4 T (Varian, CA) with a phantom (2cm diameter ball filled with deionized water). The 1H MRI was acquired using 2D GEMS sequence (TR/TE=100/4ms, FA=20°, FOV=30mm×30mm, matrix=128×128) after power calibration and B0 shimming. The 2H FID (TR=300ms, 20 number of averages (nt), spectral width (sw)=4006.4Hz, acquisition time (at)=44ms, 200µs hard pulse at Ernst pulse flip angle) and 17O FIDs (TR=50ms, nt=100, sw=4006Hz, at=44ms, 200µs hard pulse) were acquired with single-pulse-acquire sequence as a control. Then interleaved 2H- 17O data was obtained with a modified single pulse sequence shown in Figure 2. Total 20 2H-FIDs (nt=1) and 20 17O-FIDs (nt=5) were acquired with TR=50ms and identical for other parameters according the schematic. All FIDs acquired with original and modified sequences were processed in the same way and the interleaved scans were averaged to compare with the control 2H or 17O signals.
Results
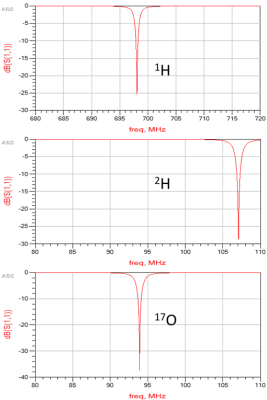
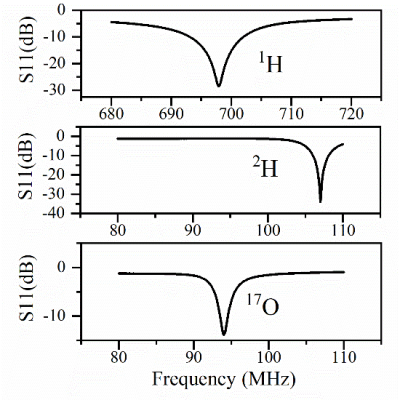
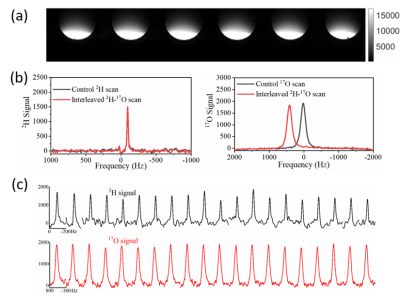
Figure 3 shows the simulated reflection coefficients at 1H, 2H, and 17O frequencies obtained from Keysight’s ADS RF simulation software, indicating great performance of the new coil design without loading. Figure 4 shows the actual S11 measurement results of a prototype coil with loading, which was built with optimal components determined in the simulation, indicating a better S11 for 1H, similar S11 for 2H, and slightly worse S11 for 17O as compared to the simulation, perhaps caused by the loading effect of a large water phantom. Using S11 parameters, the Quality factors were determined as 23.3 for 1H, 20.6 for 2H, and 20.6 for 17O.The phantom test results are summarized in Figure 5. High-quality proton imaging as shown in Figure 5(a) was obtained. Figure 5(b) displays averaged 2H and 17O signals from the interleaved scan, which matches well with the 2H and 17O signals acquired independently from the control experiments. The averaged interleaved 17O signal had a ~400 Hz shift from the control signal, which was due to the inaccurate transmitter-frequency setting in the sequence. The slight difference in the signal height was likely caused by this frequency offset. This can be easily corrected in future studies. Figure 5(c) shows arrayed 2H and 17O spectra from interleaved scans. The top panel is the deuterium spectra while the bottom panel shows the 17O spectra. The 2H signal fluctuated more than that of 17O signal because the later one had 5 times more signal averaging.
Discussion and Conclusion
A novel tri-frequency surface coil was developed and evaluated in this study. It demonstrates proficient reflection coefficients and loaded quality factors as tested on the network analyzer. A phantom study was conducted to validate the new coil for 1H, 2H, and 17O MRI/MRS at 16.4T. The results suggest that the same coil can be used to obtain not only the high-fidelity 1H anatomic information but also interleaved dynamic measurements of both 2H and 17O signals; thus, has great potential for efficient interleaved metabolic imaging of multiple critical metabolic measures, for instance, cerebral metabolic rate of glucose and TCA cycle rate using the dynamic 2H signal and cerebral metabolic rate of oxygen and blood flow using the dynamic 17O signal (see cited review article3 ).Acknowledgements
This work was supported in part by NIH grants of R01 MH111413, R01 CA240953, U01 EB026978, S10 RR025031, P41 EB027061 and P30 NS076408.References
1 Lei, H., Zhu, X. H., Zhang, X. L., Ugurbil, K. & Chen, W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med 49, 199-205, doi:10.1002/mrm.10379 (2003).
2 Zhu, X.-H. et al. In vivo 17O NMR approaches for brain study at high field. NMR in Biomedicine 18, 83-103, doi:10.1002/nbm.930 (2005).
3 Zhu, X.-H., Lu, M. & Chen, W. Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. Journal of Magnetic Resonance 292, 155-170 (2018).
4 Kan, S., Fan, M. & Courtieu, J. A single‐coil triple resonance probe for NMR experiments. Review of Scientific Instruments 51, 887-890, doi:10.1063/1.1136352 (1980).
5 Schnall, M., Subramanian, V. H., Leigh Jr, J. & Chance, B. A new double-tuned probed for concurrent 1H and 31P NMR. Journal of Magnetic Resonance (1969) 65, 122-129 (1985).
6 Zhu, X. H., Merkle, H., Kwag, J. H., Ugurbil, K. & Chen, W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 45, 543-549 (2001).
7 Alecci, M. et al. Practical design of a 4 Tesla double-tuned RF surface coil for interleaved 1H and 23Na MRI of rat brain. Journal of Magnetic Resonance 181, 203-211 (2006).
8 Pang, Y. et al. A dual-tuned quadrature volume coil with mixed λ/2 and λ/4 microstrip resonators for multinuclear MRSI at 7 T. Magnetic resonance imaging 30, 290-298 (2012).
9 Yan, X., Xue, R. & Zhang, X. A monopole/loop dual-tuned RF coil for ultrahigh field MRI. Quantitative imaging in medicine and surgery 4, 225 (2014).
10 Rutledge, O., Kwak, T., Cao, P. & Zhang, X. Design and test of a double-nuclear RF coil for 1H MRI and 13C MRSI at 7T. Journal of Magnetic Resonance 267, 15-21, doi:https://doi.org/10.1016/j.jmr.2016.04.001 (2016).
11 l Mispelter, J., Lupu, M. & Briguet, A. NMR probeheads for biophysical and biomedical experiments: theoretical principles & practical guidelines. (Imperial College Press, 2006).
12 Zhang G, Z. W., Zhu X.H, Wiesner H, Wang T & Chen W. A dual-frequency surface coil design comprised of a single loop for both proton and deuterium magnetic resonance imaging at 16.4T in Proceedings of the 28th meeting of ISMRM 28, p.4106 (2020).
Figures