1812
Interleaved 31P MRS and 1H dual-echo GRE B0 map pulse sequence for 7T Terra scanners1Wolfson Brain Imaging Center, University of Cambridge, Cambridge, United Kingdom
Synopsis
In this abstract, we demonstrate a framework for interleaving 1H imaging and 31P spectroscopy on the Siemens 7T Terra platform. We show that this can be done without making hardware modifications and demonstrate the concept with interleaved B0 map acquisition with 31P-MRS on a phantom and the quadriceps of a healthy volunteer.
Introduction
Many multinuclear MRI scans are done using dual tuned coils[1]. This makes initial localization straight forward. However, if one could interleave 1H and X-nuclear acquisitions within the same sequence this could have many advantages. In this abstract, we demonstrate a framework for interleaving 1H imaging and 31P spectroscopy on the Siemens 7T Terra platform. We show that this can be done without making hardware modifications (unlike on Magnetom 7T scanners [2]) by making appropriate changes to the “receive coil select” entries in the protocol and in ADC events for the 2nd nucleus. To demonstrate the concept, we acquire dual-echo FLASH B0 maps in the TR fill time of a 31P-MRS pulse sequence. These can be used during image reconstruction to provide a high SNR shot-to-shot frequency correction even when the 31P SNR is too low to compute this robustly from the data.Methods
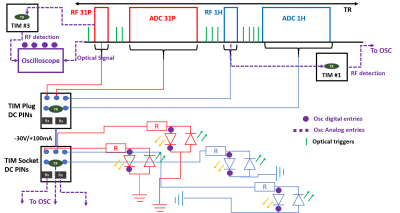
We began by looking for published examples of interleaved pulse sequences for 7T Terra, but found none. Thus, we implemented a sequence (Figure 2) that combines 1H and 31P FIDs with a recognizable pattern of gradients and optical trigger outputs at each TR.We performed experiments to verify that the DC logic pins on the TIM sockets switched with appropriate timing to ensure protection of our intended RF coil for a range of sequence protocol parameters. The setup is described in Figure 2. We prepared a Matlab(R2020b, Mathworks Inc) script that reads the unit test output and the oscilloscope values to verify (a) that every pulse transmitted on 1H or 31P has appropriate coil DC logic; and (b) to compare the switching times recorded in the unit test output against the actual on-scanner switching times to ensure proper ring-down.
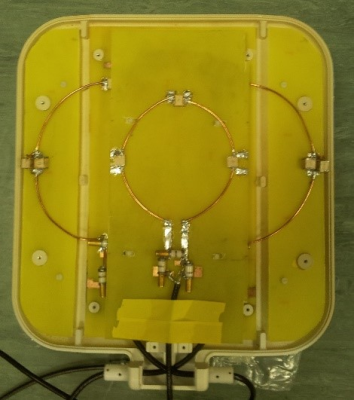
Having satisfied ourselves that it was safe, data was acquired using a surface coil comprising a 15cm quadrature pair of 31P Tx/Rx loops and a 10cm 1H loop in the center (Figure 1) on a Siemens 7T Terra MRI scanner.
Coil hardware protection tests consisted of making ourselves sure that transmit and receive would not happen at the same time during real experiment on a real coil. For this, a circuit has been built (Figure 2) with 4 branches, each branch corresponding to 1H-Tx, 1H-Rx, 31P-Tx and 31P-Rx DC logic states and the signal going through the circuit was monitored with an oscilloscope (Picoscope, 3406D MSO). A “coil file” was created so that the four Tx/Rx states could be identified from the TIM socket DC logic voltages. We implemented an Interleaved 1H-imaging and 31P-MRS sequence that records B0 maps and 31P-FIDs within the same sequence. We tested this first on a phantom with TR 1s, matrix size of 128x128 mm2, 5 mm slice thickness, FOV 250mm, bandwidth of 5000kHz. B0 maps were acquired with a dual-echo method with TEs of 3.49ms and 8.26ms.
We then acquired data in vivo from the quadriceps of a healthy volunteer with same parameters except TR that was reduced to 100ms.
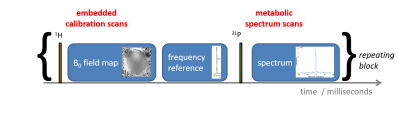
Figure 3 illustrates the sequence diagram.
Data processing
Coil hardware protection test data was processed automatically using scripts in Matlab based on logged Picoscope output, the prescribed MRI protocol and corresponding simulated sequence timings. We implemented a script that automatically computes the switching times between transmit and receive for 1H and for 31P, and that compares this against output from the sequence unit test.
Phantom and in vivo data were processed offline from raw data files in Matlab.
Finally, we implemented an ICE program that reconstructs separate DICOM spectroscopy series for 1H MRS and 31P MRS in our interleaved sequence online on the scanner as a proof-of-concept for fully online interleaved scanning.
Results
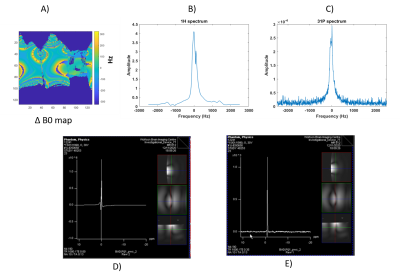
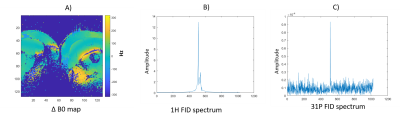
Figure 2 shows the timing sequence diagram synchronized with the oscilloscope output monitoring while sequence was running. It has been validated with a dummy coil file that transmit and receive never happen at the same time. Switching times were measured experimentally at 25us for switching from receive to transmit, and 35us from transmit to receive. This compares to timings reported in the pulse sequence unit test of 80us and 40us respectively.Figure 4 shows validation of the sequence in a phantom. We were able to retrieve B0 maps and 31P spectra in a single scan.
Figure 5 shows in vivo data acquired on the quadriceps of a healthy volunteer at 7T.
Discussion
These preliminary results show the feasibility of interleaved 1H/31P acquisitions on a 7T Terra Siemens MRI scanner. So far, we have implemented an interleaved 1H FID/31P FID sequence and a 1H GRE/31P FID sequence.We verified using a digital oscilloscope and two RF detectors that all transmit pulses (1H and 31P) were played out with the coil in the correct “transmit” DC logic mode. Switching times reported by the Siemens IDEA unit test agree with experiment to within 15-45 us.
We are now preparing a Sequence Building Block that can be used to insert interleaved MRS (FID and 3D-UTE-CSI) into an arbitrary imaging sequence. We plan to use this for studies in the liver with the NICI project and for cardiac 31P-MRS. If you wish to use this sequence, we would be happy to share subject to the usual formalities.
Acknowledgements
Jabrane Karkouri is funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 801075.
Christopher T. Rodgers is funded by the Wellcome Trust and Royal Society [098436/Z/12/B]. We acknowledge the NIHR Cambridge Biomedical Research Centre and the MRC Clinical Research Infrastructure Award for 7T research.
We also acknowledge the help from Alexandra Olaru, Iulius Dragonu and Thomas Benner from Siemens Healthcare.
References
[1] Choi, Chang-Hoon, et al. "The state-of-the-art and emerging design approaches of double-tuned RF coils for X-nuclei, brain MR imaging and spectroscopy: A review." Magnetic resonance imaging (2020): S0730-725X.
[2] Meyerspeer, Martin, et al. "Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner." Magnetic resonance in medicine 76.5 (2016): 1636-1641.
Figures