1806
High-Resolution Sodium Imaging Using Anatomical and Sparsity Constraints for Denoising and Recovery of Novel Features1Department of Electrical and Computer Engineering, University of Illinois, Urbana-Champaign, Urbana, IL, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois, Urbana-Champaign, Urbana, IL, United States, 3Center for Magnetic Resonance Research, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Quantitative sodium MRI is a unique tool for assessing tissue viability noninvasively. A major obstacle to widespread clinical applications of sodium MRI is low sensitivity, which leads to low spatial resolution even with long scan times. We present a novel method for reconstruction of high-resolution sodium maps from noisy, limited k-space data. The proposed method has been validated using simulated and experimental data, producing high-SNR and high-resolution tissue sodium concentration maps. These high-resolution maps were also shown to improve the detection of tumor responses to radiation therapy.
Introduction
Tissue sodium concentration (TSC) has long been recognized as a quantitative indicator of ion homeostasis that reflects tissue viability.1-3 Quantitative sodium MRI techniques have been developed and used to measure TSC in the human brain non-invasively, successfully detecting tumor responses to therapy4,5 and neural cell death in Alzheimer’s disease6.However, clinical applications of sodium MRI remain impeded by several longstanding technical challenges, including low sensitivity, fast biexponential T2 relaxation, long data acquisition time and low spatial resolution.3,7 Significant advances have been made in the past decades to address these challenges, from hardware development8,9 to novel pulse sequences10-15. However, the capabilities of sodium MRI at 3T are still rather limited, with nominal spatial resolution around $$$5\times5\times5$$$ mm3 and data acquisition time on the order of 10 minutes.13
Constrained reconstruction methods, such as compressed sensing16,17 and anatomically constrained reconstruction18,19, have recently emerged to enhance SNR and resolution and reduce artifacts in sodium MRI. Although compressed sensing has been used to remove or reduce noise-like artifacts, its practical performance has been limited due to low SNR and limited k-space coverage of the acquired data. Constrained reconstruction incorporating anatomical prior information from high-quality proton image can also enhance SNR and spatial resolution for image features evident in the companion proton image, but with a limited capability to reconstruct sodium-dependent novel features (e.g., lesions). In addition, anatomically constrained reconstruction is known to be sensitive to inter-scan subject motion, which affects their robustness needed for practical applications.
We have developed a new method for reconstruction of high-quality sodium images from noisy, limited k-space data in the presence of inter-scan subject motion. The new method has been tested using simulated and experimental data from human subjects, producing significantly better results than the existing methods. The high-resolution sodium concentration maps obtained using our new method were also shown to improve the detection of tumor responses to radiation therapy.
Methods
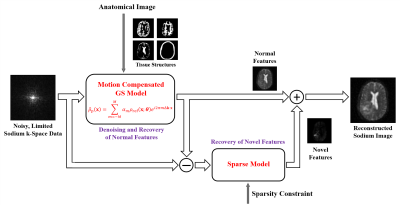
Our proposed method, illustrated in Fig. 1, synergistically integrates motion-compensated constrained reconstruction with compressed sensing for reconstruction of high-quality sodium images from noisy, limited k-space data. The key features of our method include: (a) use of anatomical prior information for denoising and resolution enhancement, (b) built-in capability for detection and correction of inter-scan subject motion, and (c) use of sparsity constraints for effective recovery of sodium-dependent novel features.More specifically, our proposed method represents the desired sodium image $$$\rho(\boldsymbol{x})$$$ using the following model:
$$\rho(\boldsymbol{x})=\sum_{m=-M}^{M} \alpha_m \rho_{\mathrm{ref}}(\boldsymbol{x};\boldsymbol{\theta})e^{-i2\pi m\Delta \boldsymbol{k\cdot x}}+\rho_{\mathrm{s}}(\boldsymbol{x}),\\
\mathrm{subject~to~}\|\Phi(\rho_{\mathrm{s}}(\boldsymbol{x}))\|_1\le\varepsilon,$$
where $$$\rho_{\mathrm{ref}}(\boldsymbol{x};\boldsymbol{\theta})$$$ represents a motion-compensated reference, $$$M$$$ the order of the generalized series (GS) model20, $$$\alpha_m$$$ the GS coefficients, $$$\rho_{\mathrm{s}}(\boldsymbol{x})$$$ an image component which is forced to be sparse under sparsifying transform $$$\Phi$$$. As compared with the conventional Fourier model, the proposed model enables the effective use of a priori information for denoising and resolution enhancement. As compared with the modern compressed sensing model, the proposed model enables better use of the measured data and the sparsity constraint for recovery of novel image features by modeling the “prior” and “novel” components separately.
To generate the reference $$$\rho_{\mathrm{ref}}(\boldsymbol{x};\boldsymbol{\theta})$$$, we first segment the proton image into different tissue compartments, then estimate the average sodium concentration of each tissue compartment
$$\{\hat{c}_r\}=\arg\min_{\{c_r\}}\left\|d(\boldsymbol{k}) - \Omega\mathcal{F}\left\{\sum_{r=1}^{R} c_rM_r(\boldsymbol{x})\right\}\right\|_2^2.$$
Then compartmental concentration variations are reconstructed using the GS model
$$\{\hat{\alpha}_m\}=\arg\min_{\{\alpha_m\}}\left\|d(\boldsymbol{k}) - \Omega\mathcal{F}\left\{\sum_{m=-M}^{M} \alpha_m\left(\sum_{r=1}^{R} \hat{c}_rM_r(\boldsymbol{x})\right)e^{i2\pi m\Delta \boldsymbol{k\cdot x}}\right\}\right\|_2^2.$$
To avoid potential image reconstruction artifacts due to inter-scan subject motion, our model also includes a built-in capability for motion estimation and correction.
We finally use sparsity constraint to recover the novel features by solving the following problem:
$$\hat{\rho}_{\mathrm{s}}(\boldsymbol{x})=\arg\min_{\rho_{\mathrm{s}}(\boldsymbol{x})}\|d(\boldsymbol{k})-\Omega\mathcal{F}\left\{\hat{\rho}_{\mathrm{ref}}(\boldsymbol{x};\boldsymbol{\theta})+\rho_{\mathrm{s}}(\boldsymbol{x})\right\}\|_2^2 + \lambda\|\Phi(\rho_{\mathrm{s}}(\boldsymbol{x}))\|_1.$$
Our method has a distinct advantage over the conventional sparsity-constrained reconstruction methods for solving the sodium imaging problem because of its ability to “remove” the “prior” component so that the sparsity constraint is applied to $$$\rho_{\mathrm{s}}(\boldsymbol{x})$$$ only. Since $$$\rho_{\mathrm{s}}(\boldsymbol{x})$$$ is much sparser than the overall image $$$\hat{\rho}_{\mathrm{ref}}(\boldsymbol{x};\boldsymbol{\theta})+\rho_{\mathrm{s}}(\boldsymbol{x})$$$, the sparsity constraint is more effective for recovery of the sodium-dependent novel features that are not present in the companion proton image.
Results
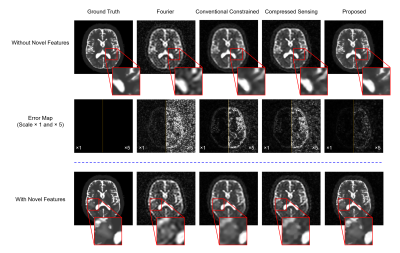
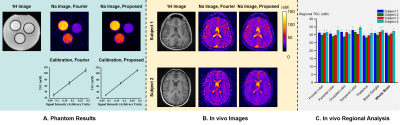
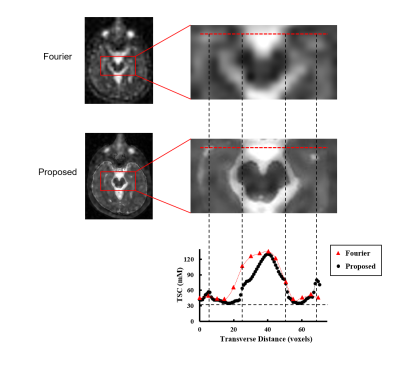
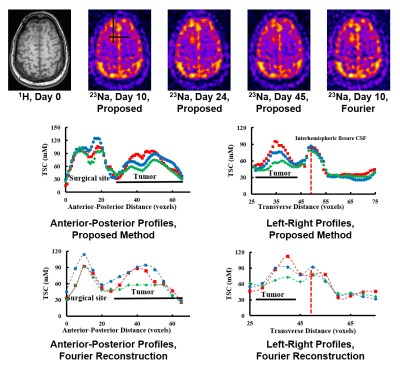
Our method has been validated using simulated and experimental data, producing high-SNR and high-resolution TSC maps. Figure 2 shows reduction in noise level and improvement in spatial resolution provided by our method in the presence of novel features in a Monte-Carlo simulation study. Figure 3 shows some representative high-quality reconstructions from phantoms and healthy subjects. Linear calibration curves with small variances from the phantom data and reproducible TSC values across different brain regions and different subjects were obtained. Figure 4 shows the capability of our method to resolve small anatomical structures and to reduce partial volume effects from surrounding CSF. Figure 5 shows high-resolution TSC changes in response to treatment in a tumor patient. The TSC decline detected by the proposed method indicated the return of infiltrated brain tissue to normal cell packing.Conclusions
A novel method for high-resolution sodium imaging has been proposed to provide an effective solution to the low resolution and low SNR problems that have limited clinical applications of sodium imaging. Simulated and experimental results have successfully demonstrated the feasibility of achieving high-quality TSC maps using the proposed method. The reduction in partial volume effects was shown to improve the detection of tumor responses to radiation treatment.Acknowledgements
The authors acknowledge Dr. Ian C. Atkinson and Dr. Aiming Lu for useful discussions about image reconstruction from twisted projection data and for handling p-files from GE 3T scanner. The work reported in this paper was supported, in part, by the National Institutes of Health (NIH); contract grant number: KRT RO1 CA1295531-01A1.References
1. Somjen GG. Ions in the brain: normal function, seizures, and stroke. Oxford: Oxford University Press; 2004. p 13-60
2. Madelin G, Regatte RR. Biomedical applications of sodium MRI in vivo. J Magn Reson Imaging. 2013;38(3):511-529.
3. Thulborn KR. Quantitative sodium MR imaging: a review of its evolving role in medicine. NeuroImage. 2018;168:250-268.
4. Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology. 2003;227(2):529-537.
5. Thulborn KR, Lu A, Atkinson IC, et al. Residual tumor volume, cell volume fraction, and tumor cell kill during fractionated chemoradiation therapy of human glioblastoma using quantitative sodium MR imaging. Clin Cancer Res. 2019;25(4):1226-1232. doi: 10.1158/1078-0432.CCR-18-2079.
6. Mellon EA, Pilkinton DT, Clark CM, et al. Sodium MR imaging detection of mild Alzheimer disease: preliminary study. AJNR Am J Neuroradiol. 2009;30(5):978-984.
7. Boada FE, Christensen JD, Huang‐Hellinger FR, Reese TG, Thulborn KR. Quantitative in vivo tissue sodium concentration maps: the effects of biexponential relaxation. Magn Reson Med. 1994;32(2):219-223.
8. Atkinson IC, Renteria L, Burd H, Pliskin NH, Thulborn KR. Safety of human MRI at static fields above the FDA 8T guideline: Sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J Magn Reson Imaging. 2007;26(5):1222-1227.
9. Shajan G, Mirkes C, Buckenmaier K, Hoffmann J, Pohmann R, Scheffler K. Three‐layered radio frequency coil arrangement for sodium MRI of the human brain at 9.4 Tesla. Magn Reson Med. 2016;75(2):906-916.
10. Nielles‐Vallespin S, Weber MA, Bock M, Bongers A, et al. 3D radial projection technique with ultrashort echo times for sodium MRI: clinical applications in human brain and skeletal muscle. Magn Reson Med. 2007;57(1):74-81.
11. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55(3):575-582.
12. Boada FE, Gillen JS, Shen GX, Chang SY, Thulborn KR. Fast three dimensional sodium imaging. Magn Reson Med. 1997;37(5):706-715.
13. Lu A, Atkinson IC, Claiborne TC, Damen FC, Thulborn KR. Quantitative sodium imaging with a flexible twisted projection pulse sequence. Magn Reson Med. 2010;63(6):1583-1593.
14. Atkinson IC, Lu A, Thulborn KR. Clinically constrained optimization of flexTPI acquisition parameters for the tissue sodium concentration bioscale. Magn Reson Med. 2011;66(4):1089-1099.
15. Nagel AM, Laun FB, Weber MA, Matthies C, Semmler W, Schad LR. Sodium MRI using a density‐adapted 3D radial acquisition technique. Magn Reson Med. 2009;62(6):1565-1573.
16. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195.
17. Madelin G, Chang G, Otazo R, Jerschow A, Regatte RR, Compressed sensing sodium MRI of cartilage at 7T: preliminary study. J Magn Reson. 2012;214:360-365.
18. Haldar JP, Hernando D, Song SK, Liang ZP. Anatomically constrained reconstruction from noisy data. Magn Reson Med. 2008;59(4):810-818.
19. Gnahm C, Nagel AM. Anatomically weighted second-order total variation reconstruction of 23Na MRI using prior information from 1H MRI. NeuroImage. 2015;105:452-461.
20. Liang ZP, Boada FE, Constable RT, Haacke EM, Lauterbur PC, Smith MR. Constrained reconstruction methods in MR imaging. Rev Magn Reson Med. 1992;4(2):67-185.
Figures