1803
Low field 19F imaging of perfluoroctylbromide: eliminating chemical shift effects and minimizing scalar coupling signal loss1Leiden University Medical Center, Leiden, Netherlands
Synopsis
Phantom 19F images have been obtained from perflurooctylbromide (PFOB) at very low magnetic field (2.15 MHz). The small spectral dispersion (in Hz) means that all fluorine nuclei contribute to the signal without chemical shift artifacts or the need for specialized sequences. Long turbo spin echo trains with short interpulse intervals and full 180o refocussing pulses suppress scalar coupling, leading to long apparent T2 values and highly efficient data collection. Overall, the detection efficiency of PFOB is actually higher than that of tissue protons at very low field.
Introduction
Although most 19F MRI is performed on either high field preclinical or clinical systems, the hardware required is quite specialized and the number of systems quite limited1. In this work we evaluate the potential of 19F imaging on a very simple and inexpensive low field MRI system2, in particular perfluorooctylbromide (PFOB) due to its high level of biocompatibility3. The obvious disadvantage is the lower intrinsic SNR. However, there are a number of advantageous features which partially mitigate this. The spectral dispersion (Hz) is very small, on the same order as the magnet homogeneity, and so the multiple resonances all give full signal. T1 is significantly shorter and the apparent T2 values can be very long, since we can use an extended TSE echo train with very short interpulse delays to refocus scalar coupling due to reduced SAR at low field.Methods
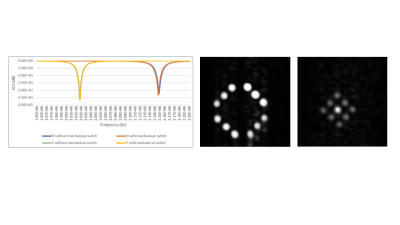
Magnet: the Halbach magnet system operating at 50 mT (2.15 MHz 1H, 2.02 MHz 19F) has been described in detail previously. Switchable MR coil: Due to the very close frequencies of 1H (2.15 MHz) and 19F (2.02 MHz) at 50 mT, the most simple approach is to mechanically switch an additional tuning capacitor in/out, the impedance matching network covers both frequencies. A solenoid coil was fabricated on a plexiglass cylinder by using a copper tape of 5 mm width and 0.07 mm thickness, 15 cm length, 9 cm diameter, 15 turns (Figure 1). Phantom: contains twenty one tubes (diameter 1 cm, length 3 cm) shown in Figure 1. The outer twelve tubes contained water doped with copper sulphate to give tissue-mimicking T1 and T2 values of 150 ms The central tube was filled with PFOB, and outer tubes with fomblin as a reference. MR measurements: T1 relaxation times were measured using an inversion-recovery sequence, T2 values were measured using a CPMG sequence. Images were acquired using a TSE sequence.Results
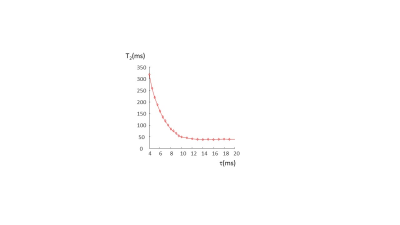
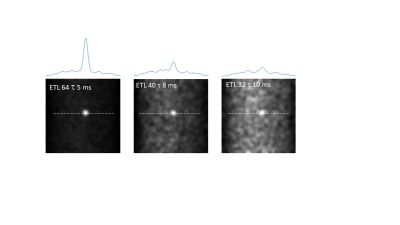
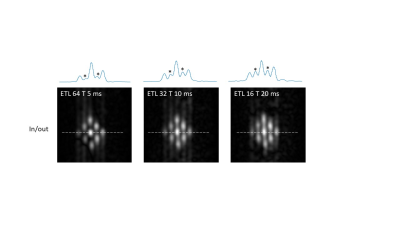
Figure 2 shows that Q values (Q~90) for the coils were essentially identical with and without the switch, and for both frequencies. A frequency shift of less than 1 kHz was introduced by the switch. 90o pulse lengths of 100 microseconds required 22 dB of attenuation from a 1 kW RF power amplifier. 1H and 19F images are also shown in Figure 2, showing no cross-talk despite the very close resonance frequencies. For the PFOB sample the T1 was measured as 720 ms. Figure 3 shows a sharp reduction in measured T2 value from the CPMG sequence as the interpulse delay increases: this is produced by the more effective refocussing of 19F-19F scalar coupling at short interpulse delays. Control experiments using water gave T2 values which did not vary with the delay. The effect of refocussing scalar coupling can be seen in Figure 4, which shows images all acquired with the same effective echo time of 160 ms (k-space covered from maximum negative to maximum positive), but with different ETLs and inter-pulse delays. The signal acquired with an ETL of 64 is ~6 times higher than that with an ETL of 32. In addition of course, an 64 x 64 image can be acquired in a single shot, whereas the one with ETL of 32 requires two shots. Alternatively, images can be acquired with a centre-out k-space coverage, which will minimize the T2-signal loss, but produces significant blurring. Figure 5 shows that for constant TE, the blurring increases as a function of interpulse delay, again showing that short values, with longer echo trains, give significantly higher SNR and fewer image artifacts.Discussion and Conclusion
The result shown here demonstrate that fluorine MRI at low field is more feasible than might be imagined just considering the available net magnetization. The chemical shift dispersion at low field is comparable, or less than, the linewidth and so all of the resonances give rise to usable signal without producing image artifacts. The T1 time is much shorter than at field strengths used for clinical or preclinical studies (T1~1200 ms at 3T5), allowing more rapid pulsing. By using very short interpulse echo times in a long TSE sequence a full 2D image can be acquired in less than a second, allowing significant signal averaging if required. The use of very short interpulse delays is also very efficient at refocussing scalar coupling evolution, resulting in an even higher imaging efficiency. Of course in vivo imaging typically involves a much lower concentration of 19F molecules compared to tissue water. However, as shown by many authors6-8, so-called “hot-spot” 19F is sufficient, in which two-dimensional projection images are acquired and overlaid on a 3D proton image. It is possible to acquire single-shot TSE fluorine images with short inter-pulse times to increase the effective T2 of PFOB. Signal averaging can then be used to increase the SNR if required.Acknowledgements
This work was funded by H2020-MSCA-ITN-ETN-2019, Horizon 2020 ERC Advanced NOMA-MRI 670629, Simon Stevin Meester AwardReferences
1. Waiczies S, Srinivas M, Flogel U, Boehm-Sturm P, Niendorf T. Special issue on fluorine-19 magnetic resonance: technical solutions, research promises and frontier applications. Magn Reson Mater Phy 2019;32(1):1-3.
2. O’Reilly T, Teeuwisse W M, and Webb A G, In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magn Reson Med. 2020;00:1-11.
3. Riess JG. Oxygen carriers ("blood substitutes")--raison d'etre, chemistry, and some physiology. Chem Rev 2001;101(9):2797-2920.
4. Colotti R, Bastiaansen JAM, Wilson A, Flogel U, Gonzales C, Schwitter J, Stuber M, van Heeswijk RB. Characterization of perfluorocarbon relaxation times and their influence on the optimization of fluorine-19 MRI at 3 tesla. Magn Reson Med 2017;77(6):2263-2271.
5. Ruiz-Cabello J, Walczak P, Kedziorek DA, Chacko VP, Schmieder AH, Wickline SA, Lanza GM, Bulte JWM. In Vivo "Hot Spot" MR Imaging of Neural Stem Cells Using Fluorinated Nanoparticles. Magnet Reson Med 2008;60(6):1506-1511.
6. Shin SH, Kadayakkara DK, Bulte JWM. Hot spot 19F MR imaging of inflammation predicts colitis-associated cancer. Cancer Res 2015;75.
7. Bouvain P, Temme S, Flogel U. Hot spot F-19 magnetic resonance imaging of inflammation. Wires Nanomed Nanobi 2020;12(6).
Figures