1797
MRI visualization of neurogenesis with ferritin and eGFP gene reporters in the intact and post-ischemic rat brain1Research Institute of Biology and Biophysics, Tomsk State University, Tomsk, Russian Federation, 2Institute of Cytology and Genetics, Novosibirsk, Russian Federation, 3Radiology, University of Washington, Seattle, WA, United States
Synopsis
The present work demonstrates feasibility of longitudinal visualization of neurogenesis in vivo with MRI gene reporter ferritin and fluorescent reporter eGFP expressed under the doublecortin promoter. Main source of the signal hypointensity in the brain neurogenic zones were mature and young neurons. Main source of the signal hypointensity in the ischemic lesion area were macrophages.
Introduction
Mechanisms of neural plasticity can be studied ex vivo in the brain preparations obtained from animals at the different stages of brain damage and restoration. Study of neurogenesis would greatly benefit from development of the tissue-specific visualization probes. Some recent publications made attempts to follow neurogenesis in vivo using fluorescent, optical, or MRI-detectable probes 1,2. Young neurons can be detected with the imaging probes expressed under the doublecortin promoter 3,4. Our study aimed to explore if overexpression of ferritin, a nontoxic iron-binding protein, under a doublecortin promoter (pDCX), can be used for non-invasive visualization of neurogenesis using MRI.Methods
Viral vectors. Ferritin heavy chain (FerrH) was expressed in the adeno-associated viral backbone (AAV) under the doublecortin promoter (pDCX), specific for young neurons, in the viral construct AAV-pDCX-FerrH. Expression of the enhanced green fluorescent protein (AAV-pDCX-eGFP) was used as an expression control.Animals. The viral vectors or PBS were injected intracerebrally to 18 adult male Sprague-Dawley rats. Three days before injection rats underwent transient middle cerebral artery occlusion (MCAO) 5,6 or sham operation.
MRI Acquisition. Animals were subjected to in vivo MRI study before surgery and on 7, 14, 21, and 28 days after viral injection using Bruker BioSpec 11.7T scanner (Bruker Biospin, Germany). The imaging protocol included Т2- and diffusion-weighted pulse sequences for detection of ischemic area and T2*-weighted multiple gradient echo pulse sequence for visualization of gene reporters in the rat brain.
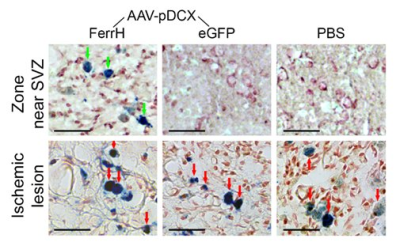
Immunohistochemistry. Brain sections obtained on day 28 after injection were immunostained for ferritin, young (DCX) and mature (NeuN) neurons, activated microglia/macrophages (CD68), and iron accumulation (Prussian Blue).
Image Analysis. All image analyses were performed using ImageJ software (NIH, Bethesda, MD, USA). Anatomically identical regions of interests (ROIs) were identified in Т2*-weighted MRI as well as in fluorescent labeled micrographs of whole brain. An average value of the signal intensity measured in ROIs on the T2*-weighted images at all imaging time points in the ipsilateral and contralateral hemispheres. The percent changes in signal relative to the time point before surgery was calculated.
Statistical analyses were carried out in Statistica 10.0 for Windows (StatSoft Inc., Tulsa, OK, USA).
Results
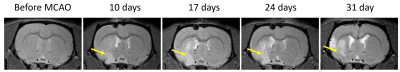
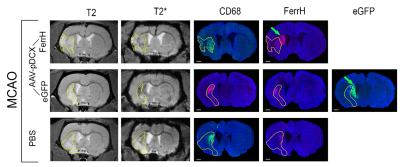
T2-weighted MRI shows development of the extensive brain lesions in the areas fed by the middle cerebral artery (Figure 1). T2* images in post-ischemic brains of animals injected by AAV-pDCX-FerrH showed two distinct zones of MRI signal hypointensity in the ipsilesioned hemisphere: in the ischemic lesion (delineated area in the Figure 2) and near lateral ventricle and subventricular zone (SVZ) (green arrows in the Figure 2). Immunochemistry showed that majority of ferritin-expressing cells in ischemic lesions were macrophages (88.1%), while ferritin-expressing cells near the lateral ventricle were mostly mature (55.7%) and young (30.6%) neurons. Ferritin as well as eGFP expression caused ~ 20% decrease in signal hypointensity in the areas near SVZ on T2*-weighted MRI at one month after intracranial injection of the viral constructs. Prussian blue staining confirmed iron accumulation in ferritin-expressing neurons near SVZ and in macrophages in the ischemic zone (Figure 3).Conclusion
Ferritin overexpression induced by injection of AAV-pDCX-FerrH to the rat brain was detected by MRI on T2* weighted images, which was confirmed by immunochemistry showing ferritin in young and mature neurons. Expression of eGFP also caused a comparable reduced MR signal intensity in T2* weighted images. Additional studies are needed to investigate the potential and tissue-specific features of the use of eGFP and ferritin expression in MRI studies.Acknowledgements
We thank the Laboratory for Viral Vector Technology & Gene Therapy, Leuven Viral Vector Core, KU Leuven, Belgium, for molecular cloning and AAV synthesis. We thank the Russian Science Foundation (project No. 18-15-00229) for funding. We deeply appreciate help of Mikhail Svetlik with MRI data processing; Anna Pishchelko with viral injections; Marina Kudabaeva with MCAO model and fluorescent intensity measurements; Yana Tumentceva with immunohistochemical staining and photographing.References
1. Couillard-Despres S, Finkl R, Winner B, et al. In vivo optical imaging of neurogenesis: Watching new neurons in the intact brain. Molecul Imag 2008; 7(1):28–34.
2. Zhang F, Duan X, Lu L, et al. In Vivo Long-Term Tracking of Neural Stem Cells Transplanted into an Acute Ischemic Stroke model with Reporter Gene-Based Bimodal MR and Optical Imaging. Cell Transplant 2017;26(10):1648–1662.
3. Adamczak J, Aswendt M, Kreutzer C, et al. Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis. Neurobiol Disease 2017;99:47–57.
4. Karl C, Couillard-Despres S, Prang P, et al. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem 2005;92(2):264–282.
5. Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20(1):84–89.
6. Gubskiy IL, Namestnikova DD, Cherkasheva EA, et al. MRI Guiding of the Middle Cerebral Artery Occlusion in Rats Aimed to Improve Stroke Modeling. Translat Stroke Res 2018;9:417–425.
Figures