1796
Measuring CMRO2 in Brain Subcortical Structures Using Dynamic 17O-MRI1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research (DKTK), Partner Site Freiburg, Freiburg, Germany, 3Dept. of Anesthesiology and Critical Care, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
Dynamic 17O-MRI with inhalation of isotope-enriched 17O2 is capable of directly quantifying the cerebral metabolic rate of oxygen consumption (CMRO2). In this work, we investigated oxygen metabolism in brain subcortical structures including thalamus, dorsal striatum, caudate nucleus and insula cortex. The determined CMRO2 values in these areas were compared with literature values obtained by PET studies. Our results show the feasibility of measuring local CMRO2 in brain subcortical structures using 17O-MRI at 3T.
Introduction
Cerebral metabolic rate of oxygen consumption (CMRO2) is a critical physiologic parameter of brain energy homeostasis and a useful indicator of the (patho)physiological state of brain tissue.1,2 Abnormal changes in CMRO2 have been reported in diseases such as tumors, stroke, Alzheimer's disease as well as other age-related pathologies in previous research.3-5 The recent development of 17O-MRI with the stable isotope 17O made it possible to perform quantitative measurements of CMRO2.In our previous 17O-MRI studies we have quantified global CMRO2 values in cortical grey matter and white matter.6-9 In this work, we provide regional CMRO2 values in brain subcortical structures including thalamus, dorsal striatum, caudate nucleus and insula cortex, and compare results with literature values obtained by PET studies.10-14
Methods
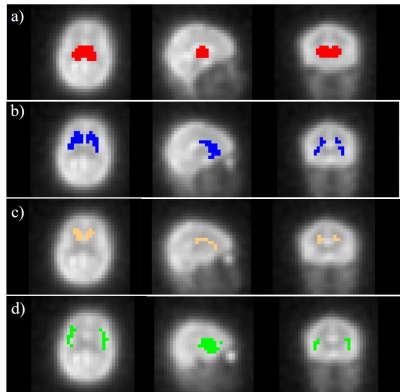
17O-MRI data sets were acquired in a dynamic 17O2 inhalation experiment using a custom-built Transmit/Receive 17O radiofrequency coil driven in circularly-polarized mode on a clinical 3 T whole body MRI system (Prisma; Siemens, Erlangen, Germany). During the inhalation experiment 70%-enriched 17O2 gas (NUKEM Isotopes GmbH, Alzenau, Germany) was administered to a healthy volunteer (male, age: 30y) in pulses of 50 mL using a demand oxygen delivery system (DODS) (Oxytron3; Weinmann, Hamburg, Germany), which is triggered by the inspiration under-pressure to deliver the rare and costly 17O2 gas efficiently. The 17O datasets were acquired before (baseline, 12 min), during (17O gas inhalation, 3 min; rebreathing, 9 min) and after (wash-out, 20 min) a delivery of 1.5±0.1 L 17O-enriched gas.A 3D ultra-short echo-time (UTE) sequence was used for 17O imaging with golden-angle acquisition pattern. The following imaging parameters were employed: TR = 9.5ms, TE = 0.51ms, BW = 360 Hz/px, number of spokes = 288k, nominal resolution = 8 mm, acquisition time TA = 44 min. Dynamic UTE data were reconstructed with conventional Kaiser-Bessel regridding and with a sliding window resulting in a temporal resolution of 1 min. Additionally, a 1H T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence was used to acquire high-resolution anatomical images (resolution: 0.9x0.9x1mm3, TI = 900ms) for image co-registration. During image co-registration, the averaged 17O images were first registered to the MPRAGE images using the FLIRT algorithm of FSL (FMRIB Software Library), followed by a non-linear registration to the MNI152 template using the FNIRT algorithm. After retrieval of the transformation matrix, the MNI structural atlas was then transformed into 17O image space and co-registered with the averaged 17O images. Regions of interest (ROIs) were defined of the thalamus, dorsal striatum, caudate nucleus and insula cortex according to the MNI atlas. Dynamic 17O data were extracted from these areas, and CMRO2 values were calculated to determine the local oxygen metabolism.
Results
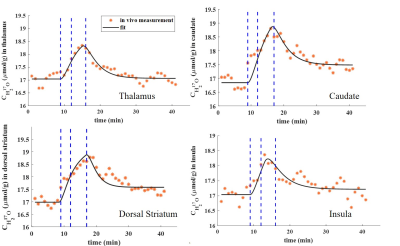
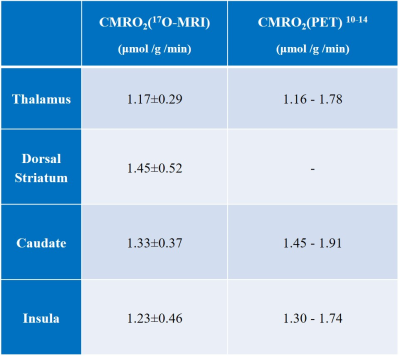
The co-registered ROIs are shown superimposed on the averaged 17O MR images (Figure 1). Figure 2 shows the resulting H217O concentration (CH217O) changes over time in thalamus, dorsal striatum, caudate nucleus as well as insula cortex during the 17O2 inhalation experiment. All measured CMRO2 values in this work are summarized in Table 1 together with literature values from PET.10-14 In the thalamus, CMRO2 value of 1.17±0.29 μmol/g/min was determined. In dorsal striatum (consisting of the caudate nucleus and the putamen) and caudate nucleus, CMRO2 values were 1.45±0.52 μmol/g/min and 1.33±0.37 μmol/g/min, respectively. For insula cortex, the measured CMRO2 value was 1.23±0.46 μmol/g/min.Discussion and Outlook
The results in this work show the possibility of measuring CMRO2 in small brain anatomical structures by using dynamic 17O-MRI at 3T. The CMRO2 value determined in thalamus was close to the previously reported values in PET studies. In caudate and insula grey matter, the measured CMRO2 values were lower than the literature values most likely caused by a systematic bias by partial volume effects with surrounding cerebral tissues. In dorsal striatum which consists of the caudate nucleus and the putamen, the determined CMRO2 value was higher than that in the caudate nucleus itself indicating a higher oxygen metabolism in the putamen which is also in line with the results from 15. In the future, more measurements are needed to evaluate the performance of 17O-MRI accurately. With an improvement in spatial resolution, 17O-MRI can potentially provide insight into oxygen metabolism in more local brain areas.Acknowledgements
Financial support from NUKEM Isotopes is gratefully acknowledged.References
[1] Hanzhang L, Feng X, Karen M. R, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex, 2011, 21 (6):1426-34.
[2] Alberto M, Michael G, Esther W, et al. Mapping the pharmacological modulation of brain oxygen metabolism: The effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. Neuroimage, 2017, 155:331-343.
[3] Aanerud J, Borghammer P, Chakravarty MM, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab, 2012, 32 (7): 1177-1187.
[4] De Vis JB, Petersen ET, Bhogal A, et al. Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. J Cereb Blood Flow Metab, 2015, 35 (6): 1015-1023.
[5] Fukuyama H, Ogawa M, Yamauchi H, et al. Altered cerebral energy metabolism in Alzheimer's disease: a PET study. J Nucl Med. 1994, 35(1):1-6.
[6] Borowiak R, Groebner J, Haas M, et al. Direct cerebral and cardiac 17O-MRI at 3 Tesla: initial results at natural abundance. MAGMA, 2014, 27 (1): 95-99.
[7] Kurzhunov D, Borowiak R, Hass H, et al. Quantification of oxygen metabolic rates in Human brain with dynamic (17) O MRI: Profile likelihood analysis. Magn Reson Med, 2017, 78 (3): 1157-1167.
[8] Kurzhunov D, Borowiak R, Reisert M, et al. Direct estimation of 17 O MR images (DIESIS) for quantification of oxygen metabolism in the human brain with partial volume correction. Magn Reson Med, 2018, 80 (6): 2717-2725.
[9] Kurzhunov D, Borowiak R, Reisert M, et al. 3D CMRO2 mapping in human brain with direct 17O MRI: Comparison of conventional and proton-constrained reconstructions. Neuroimage, 2017, 155:612-624.
[10] Pantano P, Baron JC, Lebrun-Grandié P, et al. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 1984, 15(4):635-641.
[11] Tatsuo Y, Kanno I, Uemura K, et al. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 1986, 17(6):1220-8.
[12] Ibaraki M, Miura S, Shimosegawa E, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990, 113(1):27-47.
[13] Joel P, William P, Peter H, et al. Regional asymmetries of cerebral blood flow, blood volume, and oxygen utilization and extraction in normal subject. J Cereb Blood Flow Metab. 1987, 7(1):64-7.
[14] Jun H, Hideaki F, Iwao K, et al. Regional cerebral blood flow, blood volume, oxygen extraction fraction, and oxygen utilization rate in normal volunteers measured by the autoradiographic technique and the single breath inhalation method. Ann Nucl Med. 1995, 9(1):15-21.
[15] Manouchehr V, Albert G, Nasrin I, et al. Smoking normalizes cerebral blood flow and oxygen consumption after 12-hour abstention. J Cereb Blood Flow Metab. 2015, 35(4):699-705.
Figures