1791
Sodium NMR relaxation times of human skin as potential biomarkers for Type 2 Diabetes Mellitus
Daria V Fomina1,2, Elnur G Sadykhov3, Petra Hanson4,5, Christopher J Philp1, Harpal S Randeva4,5, J Paul O'Hare4,5, Olga S Pavlova3,6, Nikolay V Anisimov6, Alexander M Makurenkov3, Yury A Pirogov3, Thomas M Barber4,5, Thomas Meersmann1, and Galina E Pavlovskaya1,2
1SPMIC, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 2NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom, 3Faculty of Physics, Lomonosov Moscow State University, Moscow, Russian Federation, 4Warwick Medical School, University of Warwick, Coventry, United Kingdom, 5Warwickshire Institute for the Study of Diabetes Endocrinology and Metabolism, University Hospitals Coventry and Warwickshire, Coventry, United Kingdom, 6Faculty of Fundamental Medicine, Lomonosov Moscow State University, Moscow, Russian Federation
1SPMIC, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 2NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom, 3Faculty of Physics, Lomonosov Moscow State University, Moscow, Russian Federation, 4Warwick Medical School, University of Warwick, Coventry, United Kingdom, 5Warwickshire Institute for the Study of Diabetes Endocrinology and Metabolism, University Hospitals Coventry and Warwickshire, Coventry, United Kingdom, 6Faculty of Fundamental Medicine, Lomonosov Moscow State University, Moscow, Russian Federation
Synopsis
Skin plays an important role in sodium regulation in the human body. As sodium interaction with macromolecules in biological tissue results in a bi-exponential T2 relaxation, a sensitive characterization of the molecular environment of the sodium ions can be made. This allows us to investigate sodium relaxation times, T2short and T2long, in human skin samples from patients with and without Type 2 Diabetes Mellitus (T2DM) using high-resolution 23Na MRS at high field 9.4T. We find that there is a significant elongation of T2long in T2DM patients. This might be indicative of disease specific skin structure changes.
Introduction
In biological tissue, sodium (23Na) experiences bi-exponential T2 relaxation with components T2long and T2short which account for 40% and 60%, respectively. This is due to the restricted and anisotropic motion created by different macromolecules, which creates conditions not only for dipole-dipole, but also for quadrupole relaxation mechanisms. Loss of tissue anisotropy and decrease of the sodium environment’s viscosity will lengthen sodium spin-spin relaxation time. Recently, a new paradigm has appeared that sodium regulation is performed by skin in the human body, along with the kidneys.1 Skin dermis is enriched with glycosaminoglycans (GAGs) in the extracellular matrix. GAGs provide a high density of negatively charged sites to bind sodium cations and make it osmotically inactive.2 Type 2 Diabetes Mellitus (T2DM) was chosen as a clinical model to investigate 23Na relaxation in human skin. It is estimated that 463 million patients worldwide have diabetes, and 90% of the cases account for T2DM.3 This work aims to demonstrate that T2short and T2long of sodium in human skin may serve as a biomarker for the presence of T2DM and provide more insight into the disease’s development.Methods
We studied 13 human skin samples from 6 control patients and 10 samples from 6 patients with T2DM in vitro. The skin samples were obtained from the abdomen and foot/leg. Skin samples had approximate dimensions 2.5*2*0.5 cm3. The necessary ethics permits were in place for this study. High resolution 23Na MRS, in particular the Carr-Purcell-Meiboom-Gill (CPMG) method, was applied to measure sodium relaxation times in an ultra-high field (UHF) 9.4T spectrometer (Avance III, UltraShield 400WB Plus, Bruker, USA) using a single channel sodium coil with 25 mm diameter (Bruker, USA) at a temperature 37°C. The Fourier Transforms of 23Na echoes were integrated in the frequency domain and the integrals were divided by a value of the receiver gain. Assuming a slow exchange between bound sodium in the skin and sodium in the extracellular fluid of the tissue, the CPMG echo train was fitted with bi-exponential decay with offset to obtain T2short and T2long (Figure 1). Constraints were applied for T2short to be less than 5 ms, following the literature.4 The data were analysed for normality using Kolmogorov-Smirnov and Jarque-Bera tests. After this, an unpaired two-tailed t-test was used to establish a difference between the two groups of patients.Results
No significant difference was found for T2short and T2long between the abdomen and foot/leg skin samples in the control group of patients (Figure 2a). Therefore, the two skin locations were combined into one control group. Regardless of age, weight, sex of the patients and other pre-existing conditions, T2DM patients had significantly longer sodium T2long than control patients (diabetic: 18.37±1.55 ms, control: 13.69±1.08 ms, P<0.001) (Figure 2b). T2short was found to be indifferent to the presence of the disease (diabetic: 0.83±0.08 ms, control: 0.86±0.13 ms).Discussion
The main finding of our work can be explained in two ways: (a) there are fewer GAGs in the extracellular matrix of skin in diabetic patients compared to the control group; (b) extracellular volume fraction is increased in the skin of patients with T2DM. In the former case, macromolecule maintenance is shifted towards proteoglycan degradation. In the latter case, sodium balance between intra- and extracellular compartments is disturbed. Both of these situations are indicators of tissue pathology that potentially opens a new insight into the development of Diabetes Mellitus. Our results also show that skin from the abdomen and foot/leg has similar viscous properties.Conclusion
Sodium T2long in human skin can serve as a biomarker for the presence of T2DM. The data of relaxation measurements on 23Na can guide a therapeutic intervention to reduce drugs’ related side effects 5 for T2DM patients. Sodium relaxation could also be monitored in the skin non-invasively during treatment to control its efficiency. Proposed methodology and obtained results may help to monitor the efficiency of intervention in patients with other diseases involving sodium homeostasis such as chronic kidney disease and cystic fibrosis.Acknowledgements
GEP and TM thank the Medical Research Council for funding (Grant No. MC_PC_15074). We would like to thank Sean James from the Arden Tissue Bank at UHCW for his help and support in the coordination and collection of the skin biopsy samples. The reported study was also funded by Russian Foundation for Basic Research and Royal Society according to the research projects No. 19-29-10015 and 20-52-10004.References
- Selvarajah V, Connolly K, McEniery C, Wilkinson I. Skin Sodium and Hypertension: a Paradigm Shift? Curr Hypertens Rep. 2018;20(11):1–8.
- Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Circ Physiol. 2004;287(1):H203–8.
- IDF. IDF Diabetes Atlas. Ninth edition 2019. 9th ed. IDF; 2019.
- Madelin G, Lee JS, Regatte RR, Jerschow A. Sodium MRI: Methods and applications. Prog Nucl Magn Reson Spectrosc. 2014;79:14–47.
- Wright EM, Loo DDF, Hirayama BA. Biology of Human Sodium Glucose Transporters. Physiol Rev. 2011;91(2):733–94.
Figures
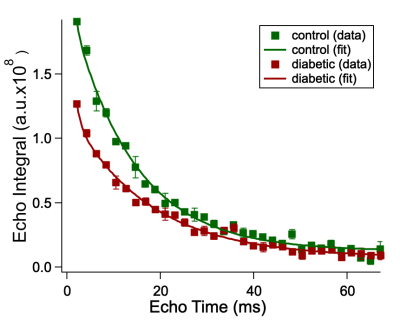
Figure 1. Sodium CPMG echo integrals of the foot/leg skin samples from control (green) and diabetic (red) patients as a function of echo time (TE). Squares represent experimental data; solid lines correspond to the calculated fitting function.
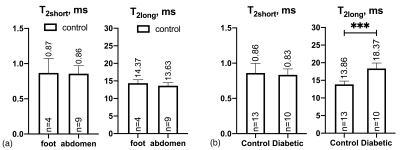
Figure 2. Sodium T2short and T2long obtained from bi-exponential fitting of CPMG (a) for samples collected from the foot/leg and abdomen from control patients and (b) for all samples from control and diabetic patients (samples from foot/leg and abdomen from control patients are presented together in one control group). ***P-value<0.001.