1788
31P dual-band pulse for selective refocusing of phosphomonoesters and phosphodiesters at low B1+ and low SAR1Department of radiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
The ratio of phosphomonoesters (PME) and phosphodiesters (PDE) is potentially an important biomarker of cancer in the body, which can be measured by 31P MR spectroscopy. Here, we introduce a dual band pulse of low B1+ amplitude for specific refocusing of these metabolite signals. This can be an alternative solution to the high power demanding adiabatic refocusing pulses used for 31P MRS in the body, upon using a multi-echo sequence.
Purpose
The ratio of phosphomonoesters (PME) and phosphodiesters (PDE), involved in membrane phospholipid metabolism, is potentially an important biomarker in tumor growth1. This ratio correlates with aggressiveness of many cancers2,3, and often changes during successful treatment4. Therefore, it can be a pivotal metric to monitor therapy response. PDE and PME can be measured by 31P MR spectroscopy (MRS) with increased intrinsic SNR at 7 Tesla. Higher SNR and insensitivity to B0 shimming can be achieved by utilizing multi-echo approaches such as the AMESING5 sequence with adiabatic pulses. This is of interest particularly in the body where B0 and B1 homogeneity is poor. However, the adiabatic pulses require significant amount of power to meet the bandwidth requirement. This subsequently results in too high SAR (specific absorption rate). An alternative solution would be to use relatively uniform B1+ fields to facilitate the use of non-adiabatic RF pulses6. With the limited B1+ available (15 μT at max in the body), we designed a low B1+ dual-band pulse which can be used in the body to selectively refocus the spins in the two frequency bands of interest. Phantom and in-vivo experiments in the brain are shown as a proof of successful operation for the dual band refocusing pulse at < 15 μT.Methods
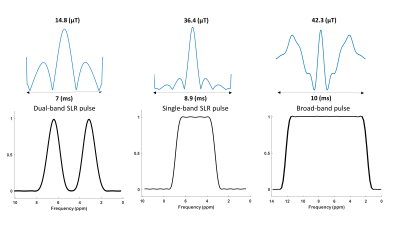
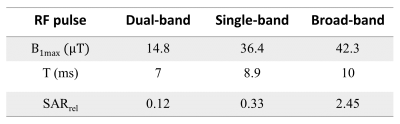
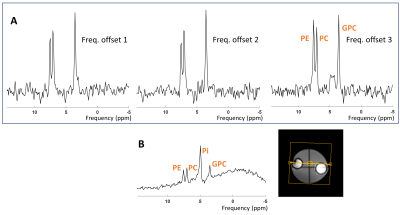
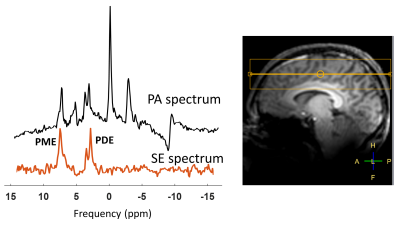
We designed a dual-band Shinnar-LeRoux (SLR) refocusing RF pulse with MATPULSE7 with a duration of 7 ms, B1+ of 14.8 μT and two narrow refocusing bands with FWHM = 166 Hz. The two refocusing bands were intended to hit the PDE and PME at 3.0 - 3.5 ppm and 6.2 - 6.8 ppm, respectively. We generated a single band RF pulse, and simulated a normal refocusing pulse used in spectroscopy from the Philips pulse archive to compare the required B1+ amplitudes and relative SAR levels to our dual-band pulse. We calculated the relative SAR of the RF pulses according to $$$\textit{SAR}_{\textit{rel}}\propto \int_0^{T_p} \! B_1^{+2}(t) \, \mathrm{d}t$$$, where Tp is the duration of the RF pulse B1+(t).MRS measurements were carried out on a 7 Tesla MR scanner (Philips, Best, NL) equipped with a double tuned 1H/31P birdcage head coil. We acquired spectra from a healthy volunteer as well as a phantom enclosing two small spheres. One of the spheres contained PMEs phosphocholine (PC) and phosphoethanolamine (PE), and PDE glycerophosphocholine (GPC), in similar concentrations (50 mM); and the other sphere contained 200 mM inorganic phosphate (Pi). We performed spin echo (SE) experiments with the dual-band pulse as refocusing pulse; sequence parameters were: TE/TR 9.98 (ms)/6000 (ms), spectral width 6400 Hz, samples 512, NSA 10, phase cycle 1, no volume localization. To evaluate the performance of the dual-band pulse, we compared it to a pulse acquire (PA) sequence with parameters: TE/TR 0.2 (ms) /1000 (ms), flip angle 45ᵒ, spectral width 5000 Hz, samples 512, number of averages (NSA) 4, phase cycles 2. A 2nd order B0 shimming (either whole phantom or whole brain) was performed prior to those experiments.
Results
The pulse shape and the frequency profile of the designed dual-band pulse are shown in Figure 1. A B1+ of around 36 (μT) would be needed to cover the ppm range including PDEs and PMEs for a complete refocusing with a single band pulse. Even more power will be needed (~39 μT) if a shorter pulse of 7 (ms) is desired. Using a broad-band profile to cover the ppm range of ~10 ppm, including the PME up to the γ-ATP signal would require a B1 amplitude of above 40 μT while still not covering the entire 31P spectrum. Relative SAR values are much lower for the dual band pulse (Table 1).Correct calibration of the frequency offset of the dual-band pulse is important as shown in Figure 2A. The third spectrum (Freq. offset 3) shows the fully refocused PDE and PME signals in the phantom. However, the difference in signal amplitude between the spin echo and pulse acquire measurement cannot be judged due to different echo time, NSAs, flip angle and TR. Refocused signals of PDEs and PMEs from spin echo sequence in vivo in the brain can be seen in Figure 3.
Discussion and Conclusion
We introduced a low B1+ (< 15 μT) dual-band pulse for selective refocusing of spin groups of PME and PDE; which are two characteristic metabolites in cancer biology. We showed 31P MRS results in a phantom and in the brain to verify the pulse shape and performance. Compared to a single band pulse with hardly sufficient bandwidth for PME and PDE refocusing, the SAR is reduced by almost a factor of 3. Because of its low power demand, the low B1+ amplitude dual-band 31P pulse has the potential to be implemented in multi-echo sequences. This pulse possibly paves way for efficient multi-echo MRSI of PME and PDE in the body, which will be investigated in future work.Acknowledgements
We acknowledge funding form Eurostars Project E!12449 IMAGINE!.References
[1] Glunde K, Bhujwalla ZM, et al., Choline metabolism in malignant transformation. Nat. Rev. Cancer, 2011; 11: 835–848.
[2] Podo F. Tumour phospholipid metabolism. NMR Biomed. 1999; 12: 413–439.
[3] van der Kemp WJM, Boer VO, et al., Increase in SNR for 31P MR spectroscopy by combining polarization transfer with a direct detection sequence. Magn. Reson. Med. 2012; 68: 353–357.
[4] van der Kemp WJ, Stehouwer BL, et al., Detection of alterations in membrane metabolism during neoadjuvant chemotherapy in patients with breast cancer using phosphorus magnetic resonance spectroscopy at 7 Tesla. Springerplus. 2014;3:634.
[5] van der Kemp WJM, Boer VO, et al., Adiabatic Multi‐Echo P Spectroscopic ImagiNG (AMESING) at 7 tesla for measuring transverse relaxation times and regaining sensitivity in tissues with short T2* values. NMR Biomed. 2013; 26:1299–1307.
[6] van Houtum Q, Welting D, et al., Low SAR 31P (multi-echo) spectroscopic imaging using an integrated whole-body transmit coil at 7T. NMR in Biomed. 2019;
[7] Matson, G.B. An integrated program for amplitude-modulated RF pulse generation and re-mapping with shaped gradients. Magn. Reson. Imaging. 1994; 12:1205-1225,
Figures