1785
Brain Metabolic Heterogeneity Revealed by Inorganic Phosphate (Pi): The Puzzling Line Broadening and Splitting of Pi Magnetic Resonance at 7T1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Despite its very small size and highly structural symmetry, the brain inorganic phosphate (Pi) signal is twice broader in linewidth or even splitting as compared to phosphocreatine signal by 31P-MRS at 7T. This puzzling phenomenon is attributed to spacial pH heterogeneity and rapid Pi diffusion between different cellular compartments, which likely prevails over other potential factors such as chemical exchange. Importantly, the Pi lineshape deconvolution by a two-component approach may provide a novel way to measure the distribution of neurons and astrocytes in brain if the assignment confirmed. Therefore this simple method may hold great promise to study neurodegenerative diseases.
INTRODUCTION
Inorganic phosphate (Pi) is required for de novo ATP synthesis (Pi + ADP → ATP) to regenerate needed energy for supporting a variety of cell functions from pumping ions across membrane to signaling cell injuries.1 As a smallest and highly symmetric molecule, Pi can rapidly diffuse between sites of ATP production and consumption in a compartmentalized cellular environment.2 Given its small size and high symmetry, one would expect a sharp narrow singlet for Pi 31P MR signal. In contrary and still puzzling, the Pi linewidth is generally broader than that of phosphocreatine (PCr), despite that the latter is far less symmetric in molecular structure and much larger than Pi in size (about 2.5-fold). This is especially the case in human brain where the Pi’s LW1/2 is typically 2-fold larger than that of PCr, as compared to the case in human calf muscle at rest (only 30% larger). With high spectral resolution under ideal circumstance, the Pi signal can be resolved into a clear doublet at ultra-high field 7T. Therefore the line-broadening phenomenon cannot be simply due to a T2-shortening effect from chemical exchange.3 The aim of this study is to present evidence for Pi line broadening and splitting in human brain at 7T, and we tentatively attribute this effect to metabolic heterogeneity due to pH difference, which could be within cells, among cells of different types such as neurons vs astrocytes/glia cells,4 and between tissues in different anatomical regions in the brain.RESULTS and DISCUSSION
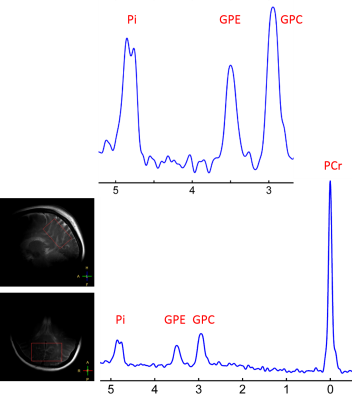
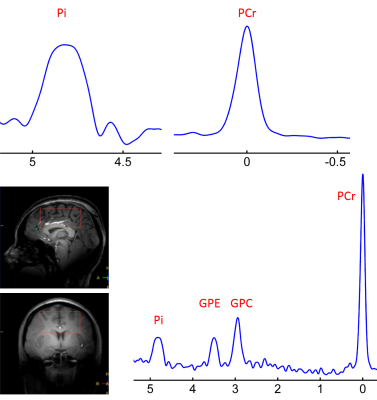
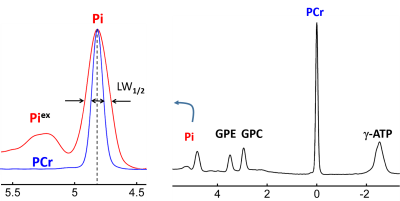
Figure 1 shows a 7T 31P-MR spectrum collected from the occipital-parietal region of a 30-yr female subject using partial volume coil. Here, the splitting of the Pi signal is clearly-resolved in the appearance of "doublet", but no such splitting or line-broadening occurs at other metabolites nearby (PCr, GPC and GPE). Of 120 subjects, we observed 7 such cases with large splitting ~0.1 ppm. In comparison, Figure 2 shows the 31P spectrum acquired from the whole brain of the same subject as in Figure 1 but using a volume coil. Here, the Pi line-broadening is visually identifiable, as referenced to PCr at 0 ppm, suggesting that the overall metabolic heterogeneity is present in the brain. Figure 3 shows an example of marginal Pi splitting, which can be separated into two peaks by a routine lineshape fitting procedure. This patterns accounts findings in 17/120 subjects. Figure 4 illustrate a more common findings of Pi line-broadening without apparent splitting (96/120 subjects). This spectrum is an average over eight healthy subjects, the LW1/2 is twice larger in Pi than in PCr (24.5 vs. 12.3 Hz). In summary, together these data reveal a metabolic heterogeneity detectable by Pi - the endogenous pH report. We speculate that the overall pH heterogeneity may reflect metabolic difference that oxygen demand is met vs not met in different tissues, and/or cell type difference on metabolism (neurons vs astrocytes).CONCLUSION
We presented spectral evidence of brain metabolic heterogeneity as revealed by the exceedingly large line broadening or even splitting at the intracellular Pi resonance. Further studies are needed to reveal the origin of this puzzling phenomenon such as regional pH difference versus pH difference between oxidative neurons and glycolytic glia/astrocytes.Acknowledgements
No acknowledgement found.References
1. Ren J, Sherry AD, Malloy CR. Efficient 31P band inversion transfer approach for measuring creatine kinase activity, ATP synthesis, and molecular dynamics in the human brain at 7 T. Magn Reson Med.2017;78(5):1657-1666
2. Gilboe DD, Kintner D, Anderson ME, Fitzpatrick JH, Emoto SE, Markley JL. Inorganic phosphate compartmentation in the normal isolated canine brain. J Neurochem. 1993;60(6):2192-203.
3. van der Kemp WJM, Klomp DWJ, Wijnen JP. 31P T2 s of phosphomonoesters, phosphodiesters, and inorganic phosphate in the human brain at 7T. Magn Reson Med. 2018;80(1):29-35.
4. Kintner DB, Anderson MK, Fitzpatrick JH Jr, Sailor KA, Gilboe DD. 31P-MRS-based determination of brain intracellular and interstitial pH: its application to in vivo H+ compartmentation and cellular regulation during hypoxic/ischemic conditions. Neurochem Res. 2000;25(9-10):1385-96.
Figures