1748
Long-term cognitive and neurological Effects of COVID-19 Illness detected by 7T MRI1Robarts research Institute, London, ON, Canada, 2Medical Biophysics, The University of Western Ontario, London, ON, Canada, 3Internal Medicine, The University of Western Ontario, London, ON, Canada, 4Parkwood Institute, London, ON, Canada, 5Infectious Diseases, The University of Western Ontario, London, ON, Canada, 6Clinical Neurological Sciences, The University of Western Ontario, London, ON, Canada, 7Paediatric Critical Care, The University of Western Ontario, London, ON, Canada, 8Medicine, The University of Western Ontario, London, ON, Canada, 9Medical Imaging, The University of Western Ontario, London, ON, Canada
Synopsis
Evidences support that the new SARS-Cov2 virus has neurological consequences in patients. Using the high resolution and sensitivity of 7T MRI, this study will determine if prolonged cognitive and neurological symptoms in patients recovered from COVID-19 illness are related to brain imaging findings. Six patients with neurological symptoms during their illness and with no respiratory symptoms for at least one month have been recruited to date. Advanced 7T MRI including susceptibility weighted imaging, diffusion tensor imaging, and chemical exchange saturation transfer imaging has been successfully acquired in four subjects with patient recruitment ongoing.
Introduction:
The novelty of the new coronavirus (SARS-Cov2), which can cause severe acute respiratory syndrome in humans, has elicited a broad scientific response. Previous studies have provided evidence that the virus can also cause neurological effects.1 These neurological effects vary from fever, headache, nausea, vomiting, loss of taste or smell to impaired consciousness, seizure and acute cerebrovascular disease. These symptoms may originate from cerebral tissue damage and may have long-term cognitive and neurological consequences even after respiratory recovery. However, it is currently unknown if cerebral tissue damage occurs as a direct effect of the virus in the brain, as a consequence of deoxygenation due to respiratory failure or from microbleeds initiated by the infection. We hypothesize that some COVID-19 patients with neurological symptoms will have long-term cognitive and neuropsychological dysfunction that may be associated with microbleeds in the brain, altered tissue pH suggestive of viral or ischemic injury, vascular damage, or alterations in tissue microstructure. Ultra-high field (7T) MRI provides unprecedented opportunities to increase image resolution and contrast to sensitively detect subtle brain pathology. The objective of this study is to determine whether lingering neurological symptoms and cognitive dysfunction in patients recovered from acute COVID-19 respiratory illness are associated with changes in the brain identified on 7T MRI.Methods:
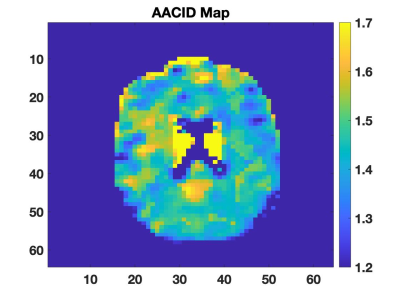
All images of the brain were acquired on a Siemens 7T MRI Plus scanner. Six patients have been recruited to-date for this ongoing study from the multidisciplinary COVID19 clinic (LUC3) in London. Included patients had neurological symptoms during their illness, were recovered for at least one month from their COVID-19 respiratory symptoms, and did not have acute psychosis, pre-existing dementia, or claustrophobia. Patients undergo cognitive testing over the phone at baseline and six months and participate in one in-person session including 7T MRI and detailed cognitive testing using the repeatable battery for the assessment of neuropsychological status (RBANS). The 7T MRI protocol includes T1-weighted MP2RAGE (700 μm iso), FLAIR (800 μm iso), 3D time of flight imaging (470 μm iso), susceptibility weighted imaging to assess microbleeds (0.1 x 0.1 x 1.3 mm3), diffusion weighted imaging (including μFA, 2 mm iso) and chemical exchange saturation transfer (CEST) imaging (3.3 mm iso) to produce pH-weighted MRI contrast using amine/amide concentration-independent detection (AACID).2 CEST images were acquired using an echo-planar-imaging (EPI) pulse sequence (TR/TE=3000/8.2 ms, ETL=28, matrix size=64 x 64, slice thickness=3.3mm, FOV=208 x 208 mm2, ipat=2, acquisition time ≈13 min) preceded by ten 100 ms RF saturation pulses (1.5 ms separation) with a B1 amplitude of 1.5 μT. CEST images were acquired at saturation frequencies ranging from 1.2 to 4.6 (Δ=0.1) ppm and 5.5 to 6.6 (Δ=0.1) ppm, and 10, 20 and 100 ppm as references with 50 images total. B0 shifts were corrected using a separate WASSR acquisition.3 Images are viewed by a neuroradiologist to detect microbleeds and other signal variations. Initial AACID-CEST MRI results are presented in this abstract.Results:
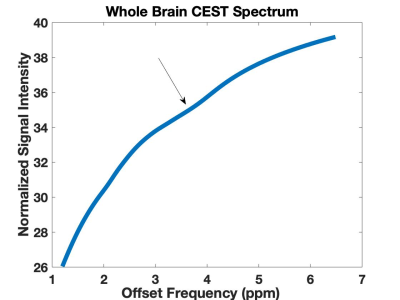
Four patients completed imaging and neuropsychological testing. Two patients showed imaging changes indicative of atherosclerosis, and one patient showed signal changes in FLAIR. AACID maps were successfully acquired in all subjects (Figure 1). Average AACID value measurements made from the whole brain region within each subject (1.48±0.03, mean ± s.d) were reproducible between subjects. Amide proton CEST effect was clearly observed at 3.50 ppm as expected (Figure 2). These preliminary results demonstrate the feasibility of obtaining pH-weighted CEST brain maps in this cohort.Conclusion:
Feasibility has been demonstrated to acquire 7T MRI in patients following COVID-19 illness, including advanced susceptibility weighted, diffusion, and CEST imaging. Further recruitment is needed to begin testing for associations between neuropsychological scores and imaging findings.Acknowledgements
Funding provided by a Western Research Catalyst Grant.References
1. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 Jun;92(6):552-555.
2. McVicar N, Li AX, Gonçalves DF, Bellyou M, Meakin SO, Prado MA, Bartha R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab. 2014 Apr;34(4):690-8.
3. Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009 Jun;61(6):1441-50.