1747
Magnetic Resonance Spectroscopy of Long COVID: Preliminary Study1Center for Clinical Spectroscopy, Brigham and Women's Hospital, Boston, MA, United States, 2Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 3Department of Neurology, Brigham and Women's Hospital, Boston, MA, United States
Synopsis
The goal of this study was to measure brain metabolites in patients recovered from COVID-19 to examine the chronic effects of COVID, also known as long COVID, which have been shown to have neurological effects. Spectroscopy in the cingulate gyrus revealed increased choline and glutamate/glutamine which may be reflective of neuroinflammatory changes due to long COVID.
INTRODUCTION
As of December 2020, there are over 74.1 million cases of COVID-19 worldwide with 97.7% of those infected surviving1. In the United States, approximately 35% of those that have recovered, have reported chronic health problems2. The most common complaints of fatigue, headaches, and chronic pain have recently been termed “Long COVID”3. These findings are similar to previous studies of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) which was found in 40% of patients that were infected with SARS in the 2000 pandemic4. ME/CFS can result from exposure to viruses and results in fatigue and cognitive effects. Indeed, studies have shown neurological sequelae in COVID patients. It is therefore imperative to examine the CNS for potential biomarkers of Long COVID. Magnetic resonance spectroscopy (MRS) is well-suited for this as numerous studies in ME/CFS have shown MRS changes in the brain in choline (Cho), N-acetylaspartate (NAA), lactate (Lac) and glutathione (GSH)6. The aim of this study was to explore the use of MRS in COVID-19 patients that were no longer acutely ill.METHODS
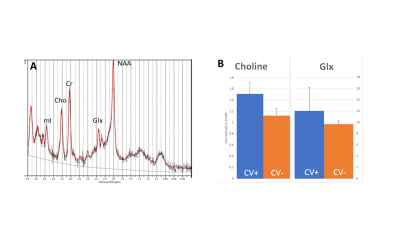
Four subjects (3 males, 1 female) with a clinical diagnosis of COVID were scanned at least 2-3 weeks after admission to the hospital. Conventional imaging was acquired on at 3T clinical MRI system and single voxel point-resolved spectroscopy (PRESS) spectroscopy (TE=30ms, TR=2000ms, voxel size 20x20x20mm, 128 averages, water reference) was acquired in the cingulate gyrus (Figure 1A). The MRS data was processed using OpenMRSLab7 for pre-processing (frequency, phase, and eddy current correction) and then post-processed using LCModel8. Metabolite concentrations of total NAA, total Cho, total creatine (Cr), glutamate/glutamine (Glx), GSH, and myoinositol (mI). MRS data from age-matched controls (n=20) in the same brain region using the same MRS acquisition and processing methods were then compared with the COVID-19 patient results.RESULTS
Results show significantly elevated Cho and Glx in the cingulate gyrus (p<0.05) as shown in Figure 1B. Despite the small number of subjects, the difference was well-powered with a Cohen’s d = 3.4 with a power of 90%. While these results are preliminary, studies are underway to acquire additional data that will be presented in the future.DISCUSSION/CONCLUSION
The pattern of changes found in these patients are similar to those found in ME/CFS6. The changes in choline and glutamate/glutamine reflect neuroinflammatory changes in the brain and could potentially serve as biomarkers for neuroinflammatory effects following COVID-19. Future studies in a large cohort will need to correlate these changes with cognitive symptoms to ensure that these MRS findings are relevant to long COVID but if successful could provide a non-invasive window into the long term effects of COVID-19.Acknowledgements
No acknowledgement found.References
1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 20(5):533-534.
2. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep 2020;69:993-998.
3. Couzin-Frankel J. The long haul. Science. 2020 Aug 7;369(6504):614-617.
4. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37.
5. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun 1;77(6):683-690.
6. VanElzakker MB, Brumfield SA, Lara Mejia PS. Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front Neurol. 2019 Jan 10;9:1033. doi: 10.3389/fneur.2018.01033. Erratum in: Front Neurol. 2019 Apr 02;10:316. Erratum in: Front Neurol. 2020 Sep 17;11:863.
Figures