1746
Imaging lung structure and function in acute COVID-19 patients with 129Xe and 1H MRI1POLARIS, Department of Infection, Immunity & Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom, 2Sheffield teaching hospitals, NHS Foundation TRUST, Sheffield, United Kingdom, 3Department of Radiology, Oxford NHS Foundation Trust, Oxford, United Kingdom, 4Department of Infection, Immunity & Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom
Synopsis
We assessed the sensitivity of a comprehensive multi-nuclear lung function-structure imaging protocol to acute changes in the lungs of patients admitted to hospital with COVID-19 lung infection. Dissolved 129Xe spectroscopic imaging and DCE 1H perfusion MRI indicate impaired gas transfer related to diffusion and microvascular perfusion limitation, whilst 129Xe ventilation MRI and 129Xe DWI indicate fairly homogenous lung ventilation and airway microstructure, apart from in areas showing clear structural abnormality on UTE/ZTE imaging (residual ground glass opacity or consolidation). The findings provide quantitative regional insight in to why patients suffer from severe breathlessness despite their lung ventilation appearing near normal.
Introduction
COVID-19 (SARS-CoV-2) literature, and clinical experience suggest that the major clinical feature of the disease is progressive hypoxaemia, dysfunctional hypoxic pulmonary vasoconstriction (HPV) and microthrombi within the lung, pulmonary circulation, heart and other organs. COVID-19 lung disease does not represent classical ARDS since in ventilated patients, lung compliance often remains near normal but oxygenation requires high inspiratory oxygen concentrations. Otherwise healthy people infected have developed coagulopathies that result in thrombosis and pulmonary embolism. Over 90% of patients hospitalized for SARS-CoV-2 infection have residual disease visible by CT imaging at time of discharge. An initial study with 129Xe MRI demonstrated abnormal regional gas transfer in acute unwell patients with COVID-19 infection (1). In this work we assess the feasibility and sensitivity of a comprehensive multi-nuclear lung function-structure imaging protocol to acute changes in the lungs of patients admitted to hospital with COVID-19 lung infection. 129Xe spectroscopic imaging and DCE 1H perfusion MRI together indicate impaired gas transfer related to diffusion and microvascular perfusion limitation, whilst 129Xe ventilation MRI and 129Xe DWI indicate fairly homogenous lung ventilation and airway microstructure apart from in areas showing clear structural abnormality on UTE/ZTE 1H MRI.Methods
Three patients were scanned who met the following inclusion criteria : (i) Hospitalised/previously-hospitalised with a diagnosis of pneumonia (chest X-ray or CT scan consistent with COVID-19 infection) (ii) Developing new onset oxygenation impairment defined as: an SpO2 ≤93% on room air AND requiring additional oxygen up to 6L/min by nasal prongs or up to FiO2 35% by mask to maintain satisfactory oxygenation. (iii) Tolerated test inhalation of 129Xe gas according to supervising clinicians judgement AND SaO2 do not fall below 80%. Two patients underwent scanning on a GE HDx 1.5T, one on a GE 450W 1.5T scanner, using flexible quadrature T-R coil (CMRS). 129Xe was polarised to >30% using a regulatory approved SEOP polariser (POLARIS, Sheffield, UK). Patient vital signs were monitored throughout. Scanner operators wore full PPE and scanners were deep cleaned for the purposes of infection control.1H MRI using an 8-element cardiac array: (i) UTE MRI was acquired with a 3D radial sequence during free-breathing with prospective respiratory bellows gating on expiration (images were reconstructed to 1.56 – 1.88 mm isotropic voxel size) (2), (ii) structural imaging with TSE propeller, (iii) inspiratory and expiratory high resolution 3D SPGR, (iv) DCE perfusion MRI with 3D time resolved SPGR imaging following injection of half dose Gadovist injected via power injector at a rate of 4ml/s followed by a 10 ml saline flush at the same rate.
129Xe MRI: (v) DW-MRI 129Xe DW-MRI was acquired after inhalation of a 1L gas mixture (550mL 129Xe + 450mL N2) using a 3D multiple b-value sequence with compressed sensing. Maps of 129Xe ADC and LmD were calculated for each voxel of the 129Xe DW-MRI as previously described (3). (vi) 3D ventilation imaging with a bSSFP sequence. (vii) 3D spectroscopic imaging of the dissolved phase peaks (dissolved xenon in blood RBC and in tissue & plasma TP) was acquired using 1L of HP 129Xe and a radial 4 echo flyback trajectory (4). Maps of gas transfer ratios (RBC:TP, RBC:Gas, TP:Gas) were produced and the amplitude of cardiopulmonary modulation of the RBC peak (RBC Osc%, (5)) was derived.
Results and Discussion
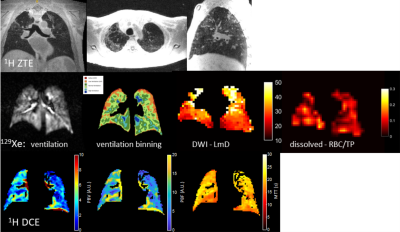
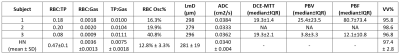
Example images are shown in figure 1 for subject 3 who exhibited significant residual lung structural abnormality (ground-glass opacity) at the end of the acute stage of infection following discharge from hospital. Both the ventilation and DWI images show some heterogeneity around the areas of structural abnormality but otherwise have global means that are not abnormal. The xenon dissolved images show impaired gas transfer with low RBC:TP ratio which maps on to the degree of perfusion impairment in DCE images. All subjects showed substantially reduced RBC:TP when compared to healthy normal ranges indicating that dissolved xenon MRI can provide a measurement of alveolar-endothelial-capillary diffusion limitation and the DCE perfusion measurements can be used to independently and directly measure any associated perfusion deficit. UTE/ZTE imaging quality shows promise for the use of 1H structural MRI as anon-ionizing follow up alternative for structural CT in COVID follow-up. Work in progress will involve following up on these patients at 6 weeks, 3 months, 6 months and 1 year to see if acute lung disease resolves or progresses to more chronic (long COVID) respiratory, interstitial or pulmonary vascular disease.Acknowledgements
This work was supported by MRC grant MR/M008894/1, GSK and GE Healthcare. The views expressed in this work are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
References
1. Li H, Zhao X, Wang Y, Lou X, Chen S, Deng H, Shi L, Xie J, Tang D, Zhao J, Bouchard LS, Xia L, Zhou X. Damaged lung gas-exchange function of discharged COVID-19 patients detected by hyperpolarized (129)Xe MRI. Sci Adv 2020.
2. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 2013;70(5):1241-1250.
3. Chan HF, Stewart NJ, Norquay G, Collier GJ, Wild JM. 3D diffusion-weighted (129) Xe MRI for whole lung morphometry. Magn Reson Med 2018;79(6):2986-2995.
4. Collier GJ, Eaden JA, Hughes PJC, Bianchi SM, Stewart NJ, Weatherley ND, Norquay G, Schulte RF, Wild JM. Dissolved (129) Xe lung MRI with four-echo 3D radial spectroscopic imaging: Quantification of regional gas transfer in idiopathic pulmonary fibrosis. Magn Reson Med 2020.
5. Ruppert K, Altes TA, Mata JF, Ruset IC, Hersman FW, Mugler JP, 3rd. Detecting pulmonary capillary blood pulsations using hyperpolarized xenon-129 chemical shift saturation recovery (CSSR) MR spectroscopy. Magn Reson Med 2016;75(4):1771-1780.
6. Chan H-F, Sjögren MP, Hughes PJC, Rodgers O, Collier GJ, Norquay G, Olsson L, Wollmer P, Wild JM, Löndahl J. Benchmarking inhaled nanoparticle measurements of airspace dimension with hyperpolarised 129Xe diffusion-weighted MRI. ERJ 2020;56:2091; DOI: 10.1183/13993003.congress-2020.2091
7. Hughes PJC, Tibiletti M, Heaton MJ, Chan H-F, Collier GJ, Austin M, Smith LJ, Lithgow J, Naish JH, Wild JM, Parker GJM. Repeatability and correlation of hyperpolarized xenon-129 and oxygen enhanced MRI parameters in healthy volunteers. Proc. Intl. Soc. Mag. Reson., 2020: Abstract 2305.
Figures