1742
Advanced magnetic resonance imaging to study brain tissue alterations in people infected with SARS-COV-21NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London (UCL), London, United Kingdom, 2Department of Brain & Behavioural Sciences, University of Pavia, Pavia, Italy, 3Brain Connectivity Center Research Department, IRCCS Mondino Foundation, Pavia, Italy, 4UCL Centre for Obesity Research, Division of Medicine, University College London (UCL), London, United Kingdom, 5Bariatric Centre for Weight Management and Metabolic Surgery, University College London Hospital (UCLH), London, United Kingdom, 6National Institute of Health Research, UCLH Biomedical Research Centre, London, United Kingdom, 7Centre for Medical Image Computing, University College London (UCL), London, United Kingdom, 8E-Health Center, Universitat Oberta de Catalunya, Barcelona, Spain, 9Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 10IRCCS Mondino Foundation, Pavia, Italy, 11Department of Electrical, Computer and Biomedical Engineering, University of Pavia, Pavia, Italy, 12Department of Medical Physics & Biomedical Engineering, University College London (UCL), London, United Kingdom, 13Department of Brain Repair & Rehabilitation, University College London (UCL), London, United Kingdom, 14Radiology, VU Medical Centre Amsterdam, Amsterdam, Netherlands, 15Neurology-Neuroimmunology Department, Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 16Department of Infection, Inflammation and Immunity, Institute of Child Health, University College London (UCL), London, United Kingdom
Synopsis
COVID-19 has now been associated with neurological symptoms, but the pathogenesis of such symptoms is unknown. Advanced quantitative MRI can provide information on alterations of brain tissue properties beyond clinically identifiable lesions. We developed a novel comprehensive scanning protocol and highly automated analysis pipeline to compare brain structure and function in people who were exposed to SARS-COV-2 infection against people who were not. Initial findings indicate that COVID-19 alters white matter microstructure and brain metabolism.
INTRODUCTION
SARS-CoV-2 is a highly infectious virus affecting a range of organs with devastating consequences1. Central Nervous System (CNS) diseases, particularly vascular disorders and altered mental status, have been reported in symptomatic hospitalised patients. Plasma biomarkers of CNS injury are elevated in patients with moderate-to-severe COVID-19. Post-mortem data have confirmed the presence of the SARS-COV-2 RNA and protein in distinct regions of the brain2. It is increasingly alarming that even people who experienced relatively mild COVID-19 report persistent symptoms, including fatigue, brain fog, anosmia and autonomic dysfunction such as orthostatic hypotension3. These persistent symptoms in otherwise healthy subjects are associated with what has now been defined as “long COVID syndrome”4.The evolving landscape of COVID-19 neurological manifestations requires detailed population-level studies in order to understand the extent of direct and indirect effects of SARS-CoV-2 on the brain5,6. Current studies are assessing possible consequences of associations between dementia genetic risk factors (apoe4) and COVID-197. Here we report initial results using a detailed quantitative MRI protocol for assessing CNS damage in people who have a history of SARS-COV-2 infection, but were not hospitalised, and compare these results with a control group.
METHODS
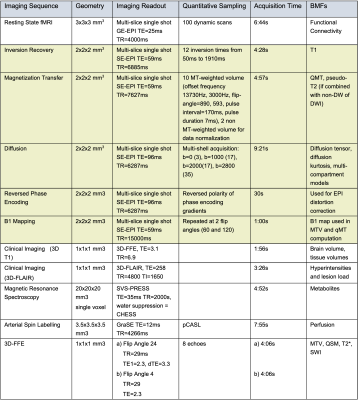
Participants: To date, fourty subjects have been recruited into this ongoing study. Of these, seven were not infected with SARS-COV-2 and were considered control subjects (CONTROL) (2 female, mean age 41 years, range 35-47 years). The remaining 33 people had polymerase chain reaction (PCR) confirmed COVID-19 (not hospitalised) or positive IgM/IgG antibodies to SARS-COV-2 (30 female, mean age 42 years, range 24-65 years) and were assigned to the COVID-19 group (COVID). Informed consent was obtained from all participants and the study was approved by a local ethics committee.MRI protocol: A Philips Ingenia CX 3T was used with a 32-channel head coil. The MRI protocol combines cutting edge methods for speed (simultaneous multi-slice echo planar imaging, compressed sensing and image denoising) with advanced biophysical modelling of MR tissue properties. The acquisition protocol has been developed in such a way that all EPI-based acquisitions have identical EPI readout. The protocol includes: Clinical 3D fluid-attenuated inversion recovery (FLAIR) sequence for detecting brain abnormalities, 3D T1-weighted acquisition for brain volume measurements, quantitative Proton Density (PD) for macromolecular total volume (MTV) calculations sensitive to the solid fraction, quantitative T2* for susceptibility weighted imaging (SWI) and quantitative susceptibility mapping (QSM) for microbleeds and tissue composition, multi-shell diffusion-weighted imaging (DWI) for microstructure assessment, resting state functional MRI (fMRI) for brain functional connectivity, quantitative magnetization Transfer (qMT) and bound pool fraction (BPF) for myelin integrity, MR spectroscopy (MRS) from a voxel positioned in the brainstem for metabolic profiling, and arterial spin labelling (ASL) for blood perfusion. The total scan time was 1 hour and the optimised parameters of the bespoke sequences are given in Table 1.
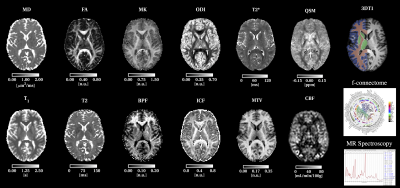
Image analysis: Data was automatically transferred from the scanner to a local XNAT server. 3DFLAIR images were assessed for hyperintensities by an experienced neuroradiologist. 3DT1-weighted scans were first processed with the NicMSLesion8 software to detect abnormalities. These masks were used for extracting Normal Appearing White Matter (NAWM) voxels as well as to perform “lesion” filling of the 3D T1-weighted scans before segmentation and registration9. All EPI acquisitions can be denoised together using the Marchenko-Pastur Principal Component Analysis (PCA)10. Pipelines for each modality are fully automated (other than MRS and fMRI) to provide biophysically meaningful features (BMFs), calculated at voxel and regional levels (Figure 1). Exploratory statistical assessment of differences between groups were performed using the two samples t-test assuming unequal variances, uncorrected for multiple comparisons. Sample size calculations were performed to estimate how large groups must be to be able to assess differences.
RESULTS
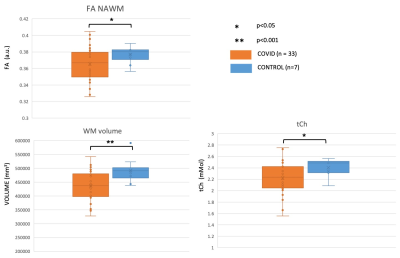
From the FLAIR radiological assessment, two subjects presented with incidental findings. Other white matter hyperintensities were consistent with small vessel ischaemia, each considered within normal range (Fazekas scores <3). Comparison of White Matter (WM), Cortical Grey Matter (CGM), and Deep Grey Matter (DGM) volumes between CONTROL and COVID showed a smaller WM volume in COVID (p<0.001) (Figure 2). Similarly, there was lower NAWM Fractional Anisotropy (FA) (p<0.01) and lower brainstem total Choline (tCh) (p<0.02) in COVID. No other statistical comparison was performed at present. Sample size calculations (confidence interval 95%, 80% power, equal group size) from these data support groups of 13 subjects each to detect differences in NAWM FA in COVID. Power calculations indicate groups of 30 subjects if we consider WM volume changes.DISCUSSION AND CONCLUSION
Our fast multi-contrast protocol allowed us to acquire a uniquely rich dataset. Preliminary analysis suggests that there are WM alterations in COVID compared to CONTROL. WM alterations indicate a reduction of WM volume and NAWM FA. Together with the decreased tCh in the brainstem, these results potentially indicate tissue damage in subjects who suffered from mild forms of COVID-19. Correlations have not yet been investigated between these changes and symptoms at the time of infection, or possible persistent symptoms. Further analysis of the data in a balanced cohort of subjects, including covariates such as age and gender, will allow us to disentangle the source of these changes and indicate whether further studies are needed to help understand the consequences of SARS-COV-2 infection in the CNS.Acknowledgements
The UK MS Society and the UCL-UCLH Biomedical Research Centre for ongoing support. Authors receive funding from the UK MS Society (#77), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541), BRC (#BRC704/CAP/CGW). FG is currently supported by PREdiCT, and investigator-initiated study at the Vall d'Hebron Institute of Oncology (Barcelona) funded by AstraZeneca. CT receives the support of a fellowship from ”la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/PI20/11760008.References
1. Hu, B., Guo, H., Zhou, P. et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol (2020). https://doi.org/10.1038/s41579-020-00459-7
2. Meinhardt, J et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. Nov. 2020. https://doi.org/10.1038/s41593-020-00758-5
3. Couzin-Frankel, J. From ‘brain fog’ to heart damage, COVID-19’s lingering problems alarm scientists. Science. July 2020. https://www.sciencemag.org/news/2020/07/brain-fog-heart-damage-covid-19-s-lingering-problemsalarm-scientists
4. Sleat, D, Wain, R, Miller, B. Long Covid: Reviewing the Science and Assessing the Risk. Tony Blair Institute for Global Change. https://institute.global/policy/long-covid-reviewingscience-and-assessing-risk. October 2020.
5. Varatharaj, A et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. The Lancet Psychiatry. October 2020; 7(10); 875-882. https://doi.org/10.1016/S2215-0366(20)30287-X
6. Paterson et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. July 2020; 143; 3104–3120. https://doi.org/10.1093/brain/awaa240
7. Chia-Ling Kuo et al. APOE e4 Genotype Predicts Severe COVID-19 in the UK Biobank Community Cohort. The Journals of Gerontology. November 2020. Series A, 75(11); Pages 2231–2232. https://doi.org/10.1093/gerona/glaa131
8. Valverde, S et al. Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage. 2017 Jul 15;155:159-168. http://doi.org/10.1016/j.neuroimage.2017.04.034
9. Prados, F et al. A multi-time-point modality-agnostic patch-based method for lesion filling in multiple sclerosis. 2016. Neuroimage 139, 376-384 (2016). http://doi.org/10.1016/j.neuroimage.2016.06.053
10. Grussu, F., Battiston, M., Veraart, J., Schneider, T., Cohen-Adad, J., Shepherd, T. M., Alexander, D. C., Fieremans, E., Novikov, D. S., and Gandini Wheeler-Kingshott, C. A. M.: Multi-parametric quantitative in vivo spinal cord MRI with unified signal readout and image denoising. NeuroImage 2020; 217, 116884.
Figures