1737
NEUROCOVID19: Impact of the Virus on the Brain1Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada, 2Rotman Research Institute, Baycrest, Toronto, ON, Canada, 3Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, ON, Canada, 4Evaluative Clinical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
NEUROCOVID19 is a longitudinal observational study to characterize the impact of the SARS-COV-2 virus on brain anatomy and function in individuals who are no longer infectious, using an extensive MRI protocol. Novel aspects include imaging of COVID-19 survivors who either self-isolated or were hospitalized, and control individuals who had cold or flu-like symptoms but tested negative for the virus. Breath-hold ultra-short TE imaging of the lung is also included to study brain-lung relationships. This report describes the study design and initial anatomical findings of the recruited participants (3 hospitalized and 23 self-isolated COVID-19 participants, 11 controls at abstract submission).
Introduction
The symptoms of coronavirus disease 2019 (COVID-19) are highly variable, ranging from minor to potentially life threatening - the latter requiring intensive critical care medical response at prevalence levels that can strain healthcare systems. Significant concern is also emerging over the potential for lingering symptoms in COVID-19 survivors.1 In particular, numerous cases of hospitalized COVID-19 patients with neurological symptoms are being reported, including deficits in sensation, cognition and consciousness, as well as vascular and inflammatory lesions observed by magnetic resonance imaging (MRI).2 Different damage mechanisms have been postulated that involve the coronavirus a) entering the brain through the olfactory and trigeminal nerves; b) entering the bloodstream to cause small hemorrhagic or ischemic strokes; or c) accessing neural tissues through a leaky blood-brain-barrier.3,4 Very little is known about how these brain effects linger or resolve - and the extent of the effects in the much larger population of COVID-19 survivors, who self-isolated while infectious. To fill this knowledge gap, we have created NEUROCOVID19: a multidisciplinary study involving neuroimaging scientists and academically-oriented clinicians, that aims to: 1. Test whether COVID-19 survivors have incident brain lesions compared to matched COVID-19 negative controls; 2. Evaluate neuroanatomy and neurophysiology, clinical, sensory, and behavioural findings when COVID-19 survivors are no longer infectious (at baseline) and at follow-up; 3. Test for multivariate relationships between these measurements across COVID-19 survivors, including associations with risk variables such as age and indices of cerebrovascular health.Methods
The study involves recruitment of COVID-19 survivors in two groups: those that were hospitalized, and those that self-isolated after testing positive (COVID+); and a control group of participants that tested negative for COVID-19 and self-isolated consequent to cold or flu-like symptoms (COVID-). NEUROCOVID19 measurements include comprehensive, state-of-the-art brain MRI at 3 Tesla (Magnetom Prisma, Siemens), including acquisitions and associated metrics probing brain anatomy and physiological function, lung MRI, olfactory and behavioural (NIH toolbox) assessments, and electroencephalography. In particular, the brain MRI protocol includes 3D T1-weighted MPRAGE, 3D T2-weighted FLAIR and SPACE imaging, susceptibility weighted imaging, diffusion tensor imaging, pseudo-continuous arterial spin labelling, resting-state BOLD functional MRI, and dynamic susceptibility contrast MRI. Lung imaging is undertaken with ultra-short echo time 3D gradient echo imaging with volumetric interpolated breath-hold examination (UTE-VIBE), including interleaved spiral k-space acquisition and iterative self-consistent parallel imaging reconstruction.5 Data are acquired in two testing sessions: an initial session undertaken nominally in a 1-2 month period after hospital discharge or leaving quarantine, with follow-up at 3 months.Results
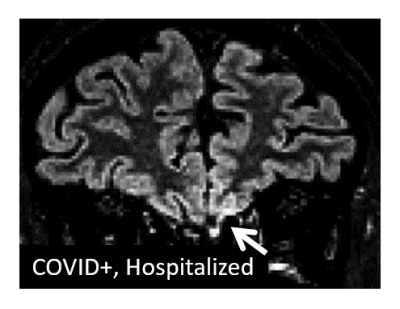
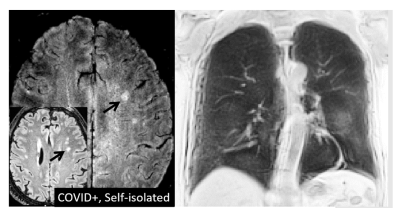
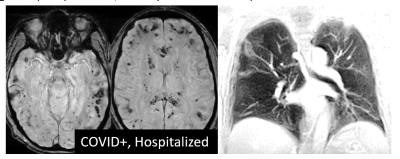
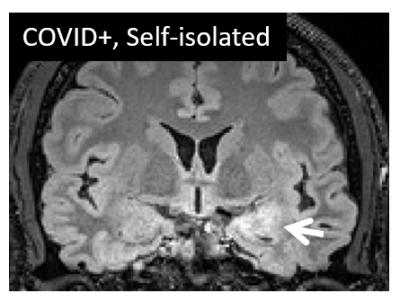
Initial session data have been obtained from 26 COVID+ cases and 11 COVID- controls at the time of abstract submission, ranging in age from 19 to 70 years (mean 43 years, 17 male). Across the COVID+ cases (3 hospitalized, 23 self-isolated), the anatomical MRI data show multiple instances of a) signal hyperintensity in the orbitofrontal cortex in the vicinity of the olfactory bulb; b) areas of infarct, ischemia, and microbleeds; and c) areas of inflammation or other neuropathy. Example images are shown in Figures 1-4. Findings were more pronounced in the hospitalized COVID+ cases, but were also well apparent in some of the self-isolated COVID+ cases. To a lesser extent, findings were seen in the COVID- controls as expected due to effects such as healthy aging. Follow-up imaging has been conducted on approximately half of all participants to date with multiple examples of brain findings persisting from the initial imaging session.Discussion and Conclusions
Our initial pilot data strongly support our hypothesis that coronavirus infection produces brain lesions in some COVID-19 survivors with and without hospitalization, over a broad age range. Recruitment and testing are ongoing, as well as analysis of all data measured in the study. Knowledge translation of our protocol and findings to radiology, neurology and COVID-19 recovery clinics will help to address on-going health needs of COVID-19 survivors, and will help to understand the mechanisms that underpin the heterogeneous brain symptoms.Acknowledgements
This project is being conducted with financial support from the Sunnybrook Foundation, the Sandra Black Centre for Brain Resilience & Recovery, and Siemens Canada Limited.References
1. Couzin-Frankel, J. The long haul. Science 2020;369(6504):614–617.
2. Ellul, MA et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783.
3. Baig, AM, Khaleeq, A, Ali, U & Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998.
4. Dando, SJ et al. Pathogens Penetrating the Central Nervous System: Infection Pathways and the Cellular and Molecular Mechanisms of Invasion. Clin. Microbiol. 2014;27(4):691–726.
5. Chassagnon, G et al. High-resolution lung MRI with Ultrashort-TE: 1.5 or 3 Tesla? Magn. Reson. Imaging 2019;61:97–103.
Figures