1734
COVID19 effects on brain tissue microstructure: Longitudinal study of self-isolated cases using diffusion MRI1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 3Hurvitz Brain Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 4Department of Psychology, University of Toronto, Toronto, ON, Canada
Synopsis
The impact of COVID19 on the brain’s microstructural integrity is still unclear. In this study, we study self-isolated COVID19 patients using diffusion-tensor and free-water imaging, based on single- and multi-shell acquisitions, respectively. We demonstrate reduced mean diffusivity and free water in both grey and white matter of patients in regions associated with vision and olfaction. At 3-month follow-up, microstructural abnormalities in patients appear to persist, and involve more brain regions, including parts of the default-mode network. Our results support the existence of measurable long-term, evolving brain deficits due to COVID19.
Introduction
It is increasingly recognized that a major cause of death and morbidity in COVID19 is infection of the central-nervous system.1 Neurological effects of COVID19 are important considerations among those that self-isolated, but remain under-investigated, particularly the more subtle microstructural brain effects that are not visible on conventional clinical scans. The only study on brain microstructure is by Lu et al., who reported, in 60 hospitalized patients reduced grey-matter (GM) mean diffusivity (MD) in patients,2 found in the insula, cingulate, precuneus, and thalamus, and attributed to the accumulation of necrotic debris in the aftermaths of neuroinflammation that impedes extracellular diffusion.3,4 However, it is unclear why no such effects were found in the white matter (WM). Moreover, it is also increasingly recognized that the COVID19 neurological effects may be prolonged beyond the period of COVID19 infection.5 In this work, we use multi-shell free-water imaging (fwDTI) in addition to conventional DTI to help clarify cellular and extracellular disease mechanisms. Moreover, we demonstrate the presence of disease effects at 3-month follow-up.Methods
All participants provided COVID19-test results prior to participating, and the controls were individuals with flu-like symptoms that tested negative for COVID19.Study Participants
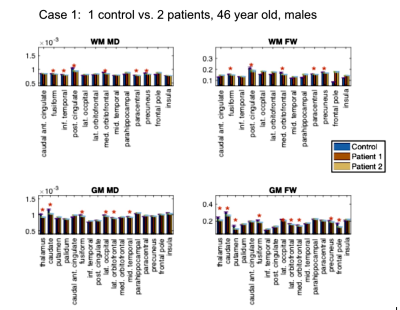
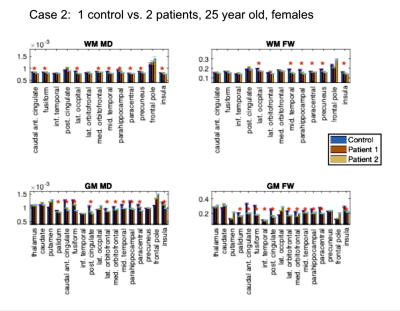
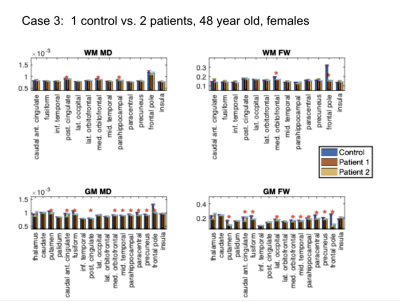
Study 1: Patients vs. controls. This analysis involves 9 participants. We compared 6 self-isolated COVI19 individuals that were otherwise healthy prior to infection against 3 controls that are each age- and sex-matched with two of the patients: (1) male, ~46 years old [normosmia]; (2) female, ~25 years old [mild anosmia]; (3) female, ~ 48 years old [normosmia].
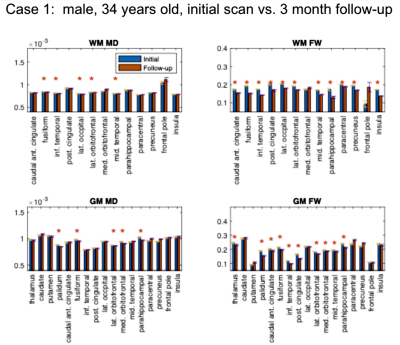
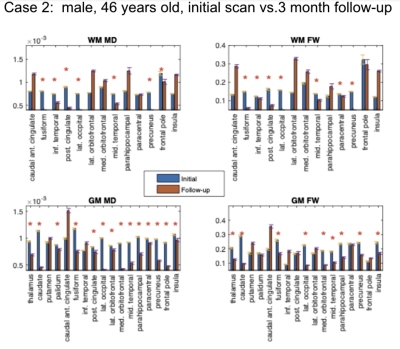
Study 2: 3-month patient follow-up. This analysis involves 3 participants that contracted COVID19. We compared the brain microstructural metrics of 4 self-isolated COVI19 individuals at the initial visit and 3-month follow-up: (1) male, 34 years old; (2) male, 46 years old; (3) male, 19 years old.
Diffusion MRI Data
Diffusion MRI data were acquired on a Siemens Prisma 3T system with 5 b=0, 34 b=700s/mm2 and 34 b=1400s/mm2 volumes at TR = 4.3 s, TE= 62ms, matrix size=96x96x60 with (2mm)3 resolution, 2-fold through-plane simultaneous multi-slice acceleration with 2 field-of-view shifts. A 1mm isotropic T1 anatomical was also acquired for each participant.
Data Processing
Fractional anisotropy (FA) and mean diffusivity (MD) were obtained using the single-compartment DTI-model fit implemented through FSL dtifit, using the b=0 and 700 shells. Free-water fraction (FW) and tissue-specific MD (MDt) were obtained by fitting the b = 0, 700 and 1400 shells to a two-compartment fwDTI model using Dipy’s multi-shell free-water code.6 These resulted in FA, MD, FW and MDt maps for all participants. Moreover, FreeSurfer reconstruction was performed, producing subject-specific GM and WM tissue parcellations used in the region-of-interest (ROI) analyses.
Statistical Analysis
ROI analysis was conducted on areas belonging to the olfactory system, which include the striatum (caudate, putamen, thalamus), precuneus and cingulate cortex.7,8 The majority of these regions demonstrated changes reported by Lu et al., consistent with the common observation of anosmia in COVID19 patients. We also included areas not involved in olfaction. Voxelwise differences between patients and controls, as well as between initial visit and follow-up, were assessed on each triplet of two patients and one control, and in each individual ROI, using unpaired t-tests corrected for multiple comparisons using the Benjamini-Hochberg procedure (thresholded at the corrected p-value that corresponds to 0.05 level significance).
Results
Study 1: In all COVID19 subjects (Fig. 1-3), we uncovered: (1) reduced FW; (2) reduced MD; (3) reduced FA (data not shown). These findings are noted for GM and WM, but also demonstrate significant inter-subject variability. Nonetheless, across all comparisons, a set of commonly affected areas are identified -- WM: posterior cingulate, medial orbitofrontal and the parahippocampal; GM: precuneus, posterior cingulate cortex, fusiform gyrus, parts of the striatum (thalamus, caudate or putamen).Study 2: The results in Fig. 4-5 are representative of the 3rd case. In all COVID19 subjects, the above microstructural abnormalities appear to be greater at follow-up. In this case, the set of commonly affected areas include-- WM: fusiform, inferior temporal, lateral occipital, lateral orbitofrontal, precuneus; GM: medial/laterial orbitofrontal, posterior cingulate cortex.
Discussion
In this work, we pioneer the application of multi-shell free-water DTI in COVID19 research. Our findings of lower FW and MD are consistent with the findings of Lu et al.,2 but are also identifiable on an individual rather than group basis. Although we did not detect significant effects in the insula, the other affected regions shared by all the patients strongly implicate the olfactory system in COVID19. We find a longitudinal reduction in FW, MD, FA and MDt at 3-month follow-up in all subjects, suggestive of persistent effects in the brain that may reflect compromised microstructure and impeded cellular/extracellular water diffusion. Similar results were also found in our patient-control comparisons, and are consistent with findings by Lu et al.,2 but the follow-up affected regions are no longer confined to the olfactory system in COVID19; rather, changes in regions of the default-mode network were also detected. Our findings suggest prolonged microstructural abnormalities even after recovering from acute COVID19, prompting future work involving follow-up sensory and cognitive measures.Acknowledgements
We thank the CIHR, NSERC, Sandra Black Centre for Brain Resilience & Recovery, and the Sunnybrook Hospital Foundation for financial support.References
1. Iadecola, C., Anrather, J. & Kamel, H. Effects of COVID-19 on the Nervous System. Cell (2020) doi:10.1016/j.cell.2020.08.028.
2. Lu, Y. et al. Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine 25, 100484 (2020).
3. Bhatt, N. et al. Role of diffusion-weighted imaging in head and neck lesions: Pictorial review. The Neuroradiology Journal vol. 30 356–369 (2017).
4. Westman, J., Grinstein, S. & Marques, P. E. Phagocytosis of Necrotic Debris at Sites of Injury and Inflammation. Front. Immunol. 10, 3030 (2019).
5. Callard, F. & Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 113426 (2020).
6. Hoy, A. R., Koay, C. G., Kecskemeti, S. R. & Alexander, A. L. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. NeuroImage vol. 103 323–333 (2014).
7. Zhang, S. & Li, C.-S. R. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage vol. 59 3548–3562 (2012).
8. Piarulli, A. et al. Ultra-slow mechanical stimulation of olfactory epithelium modulates consciousness by slowing cerebral rhythms in humans. Sci. Rep. 8, 6581 (2018).
Figures