1722
Anatomical connectivity of the anterior-posterior axis of the human hippocampus: new insights using quantitative fibre-tracking1School of Biomedical Engineering, Faculty of Engineering, University of Sydney, Sydney, Australia, Sydney, Australia, 2DVC Research, Brain and Mind Centre, University of Sydney, Sydney, Australia, Sydney, Australia, 3Sydney Imaging, University of Sydney, Sydney, Australia, Sydney, Australia
Synopsis
The hippocampus is a brain structure central to a broad range of cognitive functions including episodic memory but we know surprisingly little about how different parts of the human hippocampus anatomically connect with cortical regions to support key functions such as memory. We combined high-quality data from the Human Connectome Project with cutting-edge fibre-tracking methods to quantitatively characterise structural connectivity (SC) between the anterior/middle/posterior portions of the hippocampus and whole brain. We also mapped the distribution of endpoints within the hippocampus for streamlines connecting from cortical regions. Our results provide key contributions to ongoing efforts to characterise human hippocampal SC.
Introduction
The hippocampus is a brain structure that is central to a broad range of cognitive functions including episodic memory1. It is well-known that the primary anatomical input into the hippocampus is via the entorhinal cortex. However, tract-tracing data from rodent and non-human primate studies has shown that specific cortical brain regions bypass this canonical hippocampal pathway and directly target specific subregions within the hippocampus2,3. In addition, these direct pathways show gradients of connectivity along its anterior-posterior axis3, which suggests that different portions of the hippocampus have unique patterns of structural connectivity (SC) with cortical regions.We currently lack a detailed understanding of how different parts of the human hippocampus anatomically connect with cortical regions to support key functions such as memory. Due largely to the technical difficulties inherent to investigating SC of the human hippocampus, only a small number of studies have attempted to characterise the SC of the hippocampus with the whole brain4, and, to our knowledge, there has been no systematic examination of SC patterns along its anterior-posterior axis.
We combined high quality data from the Human Connectome Project (HCP) with cutting-edge fibre-tracking methods5 with three primary aims: to quantitatively characterise SC between whole hippocampus and whole brain (Aim 1); between anterior/middle/posterior portions of the hippocampus and whole brain (Aim 2); and to map the distribution of endpoints within the hippocampus for streamlines connecting from cortical regions (Aim 3).
Methods
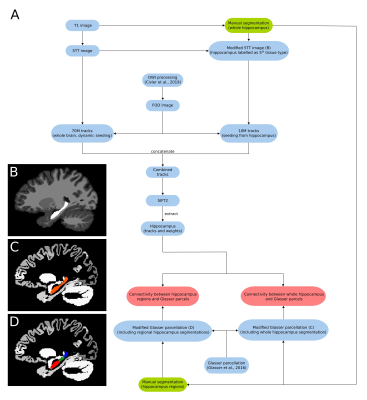
Ten subjects were selected from the HCP 100 unrelated subject database6. The image processing pipeline based on MRtrix3 is summarised in figure 1A. Diffusion pre-processing steps have been described elsewhere7. 70M tracks were generated across the entire brain using dynamic seeding. The hippocampus was manually segmented for each participant on the T1 image. The segmentation was labelled as ‘5th tissue type’ on a modified-5TT image (figure 1B) to allow streamlines to enter the hippocampus rather than terminate at the grey matter/white matter border8. Fibre-orientation distribution (FOD) data were used with the modified-5TT image to generate an additional 10M tracks seeding from the hippocampus. The 70M whole-brain tracks and 10M hippocampus tracks were combined and SIFT2 was done on the 80M track file9. Tracks (and SIFT2 weights) which had an endpoint in the hippocampus were isolated. A connectome was generated using tracks and weights from the hippocampus and a modified Glasser parcellation10 which contained the participant specific manually segmented hippocampus mask (figure 1C) in place of the automated hippocampus parcel. For the regional analysis, the manually segmented hippocampus mask was divided into anterior, middle and posterior portions using previously described methods11 (figure 1D). Each region was added to the parcellation file as its own unique parcel.Results
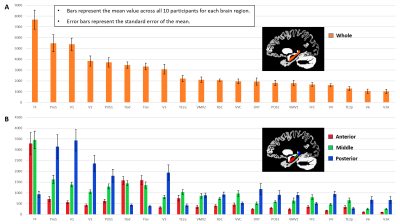
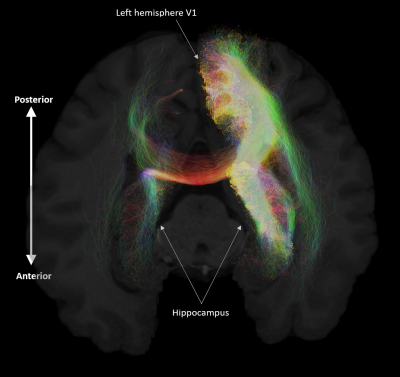
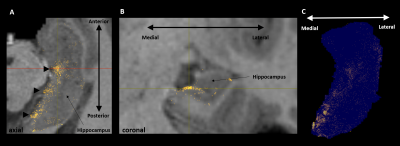
Aim 1: As predicted, medial temporal lobe (MTL; including entorhinal cortex) and subcortical structures accounted for most streamlines entering the hippocampus (62% of all streamlines). Occipital regions accounted for 15%, non-MTL temporal regions for 11%, and parietal regions for 10% of all streamlines, respectively. We observed comparatively fewer connections between the hippocampus and frontal, insula and somatosensory/motor regions. Figure 2A presents the top 20 non-MTL cortical brain regions connecting with the whole hippocampus. Aim 2: We observed striking differences in SC patterns between anterior/middle/posterior hippocampus and cortical regions. For example, the anterior 2/3 of the hippocampus showed preferential connectivity with specific brain regions in the temporal lobe. In contrast, the posterior hippocampus showed preferential connectivity with specific brain regions in the occipital and medial-parietal lobes (Figure 2B). Aim 3: Our method allowed us to visualise streamlines between the hippocampus and each cortical region (e.g. left V1 in Figure 3) and, for the first time, the location of streamline endpoints within the hippocampus. For specific cortical ROIs (e.g. left retrosplenial cortex in Figure 4), we observed clusters of endpoints localised to circumscribed regions within the hippocampus .Discussion
SC patterns generally aligned with predictions based on observations from the non-human primate literature. Medial parietal regions showed preferential connectivity with posterior hippocampus while temporal regions showed denser patterns of connectivity with anterior hippocampus. Our results suggest, however, that the human hippocampus may have denser patterns of SC with occipital brain regions than would be expected from non-human primate literature. We found strong patterns of SC between the posterior hippocampus and specific occipital brain regions associated with aspects of visual processing. This observation has implications for theories of human hippocampal function along its anterior-posterior axis12,13 and support proposals that the posterior hippocampus may have a preferential role in elements of visual perception14. Furthermore, our novel method of characterising where within the hippocampus streamlines terminate identified that brain regions implicated in elements of visuo-spatial processing appear to preferentially ‘target’ portions of the medial hippocampus. This observation supports recent proposals that the medial hippocampus is an important hub for aspects of visuospatial cognition15.Conclusion
Detailed maps of hippocampal SC will help develop more detailed and integrated models of human memory and its biological basis. Overall, our results contribute to ongoing efforts to characterise human hippocampal SC, with implications for understanding human hippocampal function in health and dysfunction in disease.Acknowledgements
No acknowledgement found.References
1. Maguire EA, Mullally SL. The hippocampus: a manifesto for change. J Exp Psychol Gen. 2013;142(4):1180-9.
2. Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol. 2007;502:810-833.
3. Insausti R, Muñoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis) Eur. J. Neurosci. 2001;14:435-451.
4. Maller JJ, Welton T, Middione M, et al. Revealing the Hippocampal Connectome through Super-Resolution 1150-Direction Diffusion MRI. Sci Rep. 2019;9:2418.
5. Tournier JD, Smith RE, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019;202:116–37.
6. https://db.humanconnectome.org/app/template/SubjectDashboard.vm?project=HCP_1200&subjectGroupName=100%20Unrelated%20Subjects
7. Civier O, Smith RE, Yeh CH, et al. Is removal of weak connections necessary for graph-theoretical analysis of dense weighted structural connectomes from diffusion MRI? NeuroImage. 2019;194:68-81.
8. Smith RE, Tournier JD, Calamante F, et al. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage. 2012;62:1924–1938.
9. Smith RE, Tournier JD, Calamante F, et al. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage. 2015;119:338-51.
10. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178.
11. Bernasconi N, Bernasconi A, Caramanos Z, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region, Brain. 2003;126(2):462–469.
12. Strange BA, Witter MP, Lein ES, et al. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655-669.
13. Poppenk J, Evensmoen HR, Moscovitch M, et al. Long-axis specialization of the human hippocampus. Trends Cognit. Sci. 2013;17:230-240.
14. Zeidman P, Mullally S, Maguire EA. Constructing, perceiving, and maintaining scenes: hippocampal activity and connectivity. Cereb Cortex. 2015;25:3836–3855.
15. Dalton MA, Maguire EA. The pre/parasubiculum: a hippocampal hub for scene-based cognition? Curr Opin Behav Sci. 2017;17:34-40.
Figures