1708
Longitudinal Brain Atlases of Early Developing Cynomolgus Macaques from Birth to 48 Months of Age1Southern Medical University, Guangzhou, China, 2University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Kunming University of Science and Technology, Kunming, China
Synopsis
In analyzing the early postnatal brain development featuring extremely dynamic imaging contrast, brain appearance, shape and size, longitudinal brain atlases with densely sampled time-points and ancillary anatomical information are of great importance, but remain absent in cynomolgus macaques, which is a highly valuable animal model for understanding human brains. To fill this critical gap, we construct the first set of spatiotemporal (4D) brain atlases and associated ancillary anatomical information with 12 time-points (i.e., 1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 36, and 48 months of age) based on 175 longitudinal structural MRI scans from 46 cynomolgus macaques.
Introduction
Due to the rapid changes in imaging contrast, brain appearance and anatomical structure during the early postnatal stages, longitudinal brain atlases with densely sampled time-points and ancillary anatomical information are of fundamental importance for analyzing the dynamic and critical brain development during this stage1,2. Many human neuroimaging studies have proved that applying the infant dedicated, age-specific atlas as a reference to normalize brain MR images is more accurate than directly using the adult brain atlas3,4,5. However, for cynomolgus macaques, which is a highly valuable animal model for understanding human brains, the existing brain atlases are mainly based on adults6,7, showing a notable lack of temporally densely sampled atlases focused on the dynamic early brain development. This study aims to fill this critical gap by constructing the first set of spatiotemporal (4D) cynomolgus macaque atlases and associated tissue probability maps (gray matter, white matter, and cerebrospinal fluid), parcellation maps, and cortex lobe maps, with totally 12 dense time-points (at 1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 36, and 48 months of age) based on 175 longitudinal structural MRI scans from 46 healthy cynomolgus macaques. This 4D atlas will be released to the public soon as a valuable resource to facilitate the macaque brain development studies in the field.Methods
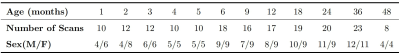
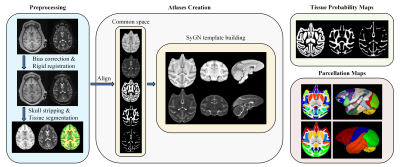
In this study, a total of 175 longitudinal scans, including T1-weighted (T1w) and T2-weighted (T2w) images, from 46 cynomolgus macaques (23 females and 23 males) were longitudinally acquired with a GE Signa HDxT 3.0T MRI scanner for the generation of atlases. The age and gender distribution are shown in Table 1. The main steps of atlas construction are illustrated in Fig. 1. For preprocessing, 1) all pairs of T1w and T2w images have undergone visual inspection for quality control; 2) N4 bias correction8 in ANTs9 was performed for intensity inhomogeneity correction; 3) rigid registration with FLIIRT in FSL10 was used to align each T2w image to its corresponding T1w image; 4) all the images were fed into the DIKA-Nets11, which is our previously proposed skull stripping method for developing macaques; 4) we generated tissue segmentation maps, including the labels of gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) for each scan by LINKS12, and further manually corrected the results. All the individual images and corresponding tissue maps were transformed in a common coordinate space together by rigidly registering them into the UNC-Wisconsin juvenile macaque atlas13. To create optimal age-specific atlases with both unbiased shape and appearance, based on both intensity and tissue labels, we employed the symmetric group-wise normalization (SyGN) atlas building algorithm14,15, which is widely exploited in building human16 and non-human primates brain atlases7. Accordingly, for each age group, we generated the age-specific intensity templates of T1w and T2w image and tissue probability maps of GM, WM, and CSF. For parcellation of the generated cynomolgus atlases, we first used the symmetric normalization algorithm17 to warp the D99 macaque brain atlas18 to the generated 48-month template, and then propagated the warped parcellation map longitudinally to each early-time atlas. Moreover, we also manually merged these parcellation maps into lobe-level, including the frontal lobe, parietal lobe, occipital lobe, temporal lobe, limbic lobe, insular lobe, and cerebellum.Results and Discussion
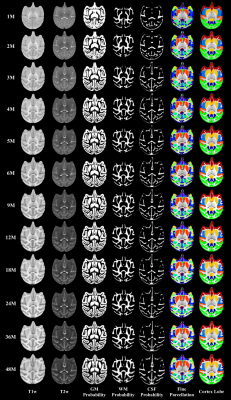
Our generated 4D atlas of cynomolgus macaque brains has 12 time-points at 1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 36, and 48 months of age, thus densely covering the dynamic early postnatal brain development. Examples of typical axial slices from the 12 age-specific atlases include T1w and T2w templates, and corresponding anatomical information, i.e., the tissue probability maps for GM, WM, and CSF, the fine parcellation label maps, and the coarse lobe label maps, as shown in Fig. 2. Note that these templates show the dynamic developmental changes in brain appearance and anatomical structure, especially in the first year. Each voxel in the tissue probability map contains a value between 0 and 1, describing the average likelihood that the voxel belongs to a tissue type.Conclusion
We have constructed the first 4D cynomolgus macaque atlas that covers the entire early brain development (from birth to 4 years of age) with densely sampled 12 time-points at 1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 36, and 48 months of age. These atlas images alongside the supporting information of tissue probability maps, fine parcellation label maps, and the coarse lobe label maps will be publicly disseminated for the community soon.Acknowledgements
No acknowledgement found.References
1. Li, G., Wang, L., Yap, P.-T., et al. Computational neuroanatomy of baby brains: A review. Neuroimage. 2019; 185: 906-925.
2. Scott, J.A., Grayson, D., Fletcher, E., et al. Longitudinal analysis of the developing rhesus monkey brain using magnetic resonance imaging: birth to adulthood. Brain Structure and Function. 2016; 221(5): 2847-2871.
3. Fillmore, P.T., Phillips-Meek, M.C., Richards, J.E. Age-specific MRI brain and head templates for healthy adults from 20 through 89 years of age. Frontiers in aging neuroscience. 2015; 7: 44.
4. Sanchez, C.E., Richards, J.E., Almli, C.R. Age-specific MRI templates for pediatric neuroimaging. Developmental neuropsychology. 2012; 37(5): 379-399.
5. Fonov, V., Evans, A.C., Botteron, K., et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011; 54(1): 313-327.
6. Frey, S., Pandya, D.N., Chakravarty, M.M., et al. An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). Neuroimage 2011; 55(4): 121-131.
7. Lv, Q., Yan, M., Shen, X., et al. Normative Analysis of Individual Brain Differences Based on a Population MRI-Based Atlas of Cynomolgus Macaques. Cerebral Cortex. 2020; 31(1):341-355.
8. Tustison, N.J., Avants, B.B., Cook, P.A., et al. N4ITK: improved N3 bias correction. IEEE transactions on medical imaging. 2010; 29(6): 1310-1320.
9. http://stnava.github.io/ANTs/
10. Jenkinson, M., Beckmann, C.F., Behrens, T.E., et al. FSL. Neuroimage. 2012; 62(2): 782-790.
11. Zhong, T., Zhang, Y., Zhao, F., et al. Domain-Invariant Prior Knowledge Guided Attention Networks for Robust Skull Stripping of Developing Macaque Brains. International Conference on Medical Image Computing and Computer-Assisted Intervention 2020. Springer, pp. 22-32.
12. Wang, L., Gao, Y., Shi, F., et al. LINKS: Learning-based multi-source IntegratioN frameworK for Segmentation of infant brain images. Neuroimage. 2015; 108: 160-172.
13. Styner, M., Knickmeyer, R., Joshi, S., et al. Automatic brain segmentation in rhesus monkeys. Medical Imaging 2007: Image Processing. International Society for Optics and Photonics, p. 65122L
14. Avants, B.B., Tustison, N.J., Song, G., et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011; 54(3): 2033-2044.
15. Avants, B.B., Yushkevich, P., Pluta, J., et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010; 49(3): 2457-2466.
16. Dong, H.-M., Castellanos, F.X., Yang, N., et al. Generating Templates and Growth Charts for School-Aged Brain Development. 2019; bioRxiv, 747352.
17. Avants, B.B., Epstein, C.L., Grossman, M., et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis. 2008; 12(1): 26-41.
18. Reveley, C., Gruslys, A., Ye, F.Q., et al. Three-Dimensional Digital Template Atlas of the Macaque Brain. Cerebral Cortex. 2016; 27(9): 4463-4477.