1706
Exogenous sex hormone effects on brain microstructure in women: a diffusion MRI study in the UK Biobank1Imaging Genetics Center, Mark and Mary Stevens Neuroimaging & Informatics Institute, University of Southern California, Marina del Rey, CA 90292, USA, Los Angeles, CA, United States
Synopsis
In women, higher levels of sex-related hormones have been associated with increased risk for age-related neurodegenerative diseases such as Alzheimer’s disease. We measured age-associated effects of exogenous sex hormones on brain white matter microstructure in pre- and post-menopausal women, using four diffusion-weighted MRI models (DTI, TDF, NODDI and MAPMRI). Estrogen therapy alone was associated with accelerated aging in white matter metrics, compared to hormone replacement therapy containing both estrogen and progestin. This effect was most evidenced by multi-shell diffusion method NODDI, compared to DTI and other advanced diffusion methods.
INTRODUCTION
Non-invasive in vivo imaging biomarkers that are associated with age and cognition have been increasingly used as proxies to assess effects of menopausal sex hormones on the brain.1,2 Previous studies conducted in women have shown that higher levels of sex-related hormones (such as estrogen) may be associated with increased risk for age-related neurodegenerative diseases such as Alzheimer’s disease.3 Positive and negative brain structural changes have been associated with estrogen fluctuations in pregnant women4 and in pre-menopausal women across the menstrual cycle5; however, we know very little about how sex hormone levels affect brain aging. We modelled age-associated effects on white matter microstructure to infer possible effects of exogenous sex hormones on the brains of pre- and post-menopausal women using diffusion-weighted MRI (dMRI). We examined the association between exogenous sex hormone exposure and aging brain white matter trajectories in women using hormone therapy (HT) or oral contraceptives (OC), relative to non-users, using 4 diffusion models, from single-shell tensor-based (DTI, TDF) to multi-shell (NODDI, MAPMRI) methods. We also accounted for differences in HT formulation (estrogen or estrogen + progestin) and investigated the link between duration of therapy, age at menopause and HT/OC onset, and white matter aging on diffusivity metrics in women.METHODS
Our sample was drawn from the population-based UK Biobank6 and included n=8,126 (HT users: n=3,033, non-users n=5,093; estrogen only HT users: n=300; combination HT users: n=98), and n=8,193 (OC users: n=7,026; non-users n=1,167) pre-menopausal and post-menopausal women aged 45-80 years. Diffusion tensor imaging (DTI) fitting was performed on the b=1000 s/mm2 shell (50 directions) using the FSL’s DTI fitting (DTIFIT) tool to create maps of tensor-derived fractional anisotropy (FA), mean (MD), radial (RD) and axial (AD) diffusivity. The dMRI data (b=1000 s/mm2, 50 directions) were also used to estimate the tensor distribution function7 to create TDF-based diffusion maps of FA (TDF-FA) – an approach that extends multi-tensor models of diffusion to describe intravoxel fibers as a probabilistic collection of tensors. The dMRI data (b=1000 and 2000 s/mm2, 100 directions) were also input into Neurite Orientation Dispersion and Density Imaging (NODDI)8 models, using the AMICO tool to obtain voxel-wise microstructural parameters representing the orientation dispersion index (OD) and intracellular (ICVF) and extracellular volume (ISOVF) fractions. Furthermore, dMRI data were input into MAPMRI9,10, which models the three-dimensional q-space signal and transforms it into a diffusion propagator using DiPy, to obtain return-to-the-origin probability (RTOP), the return-to-the-plane probability (RTPP), and the return-to-the-axis probability (RTAP) metrics. Voxel-wise statistical analysis of the images was performed using tract-based spatial statistics (TBSS).11 Average whole-brain measures were extracted for all diffusivity indices for each participant.We employed higher order fractional polynomials (FP) to characterize sex hormone effects on age-related trajectories in white matter, testing one and two-term curvilinear model using predefined set of power terms (2, -1, -0.5, 0, 0.5, 1, 2, 3) and the natural logarithm function of age. Main nuisance covariates included years of education (binarized into no college/college), waist/hip ratio, population structure (measured using the first four genetic principal components obtained from the UK Biobank’s genetic ancestry analyses)12 and the Townsend index13, a measure of socioeconomic status. We also considered participants’ history of hysterectomy and bilateral oophorectomy, and number of childbirths, as covariates. Additional model-specific covariates included duration of HT/OC usage, age at HT/OC therapy and menopause. Normalized centile curves14–16 were computed to visualize white matter aging trajectories for the white matter indices showing significant effects.
RESULTS
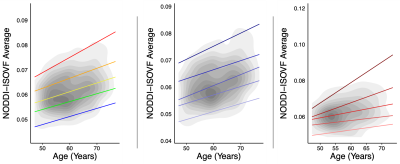
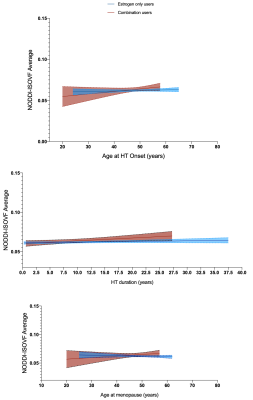
There was no significant interaction between age and HT status or OC status on diffusion metrics when including the main nuisance covariates (p>0.05). Supplemental analyses also adjusting for surgical history (hysterectomy and oophorectomy) and number of childbirths resulted in similar findings (HT/OC analyses: p>0.05). Follow-up exploratory analyses examining combined or unopposed estrogen treatment in HT users, revealed a significant association between diffusion metric NODDI-ISOVF and age, with estrogen-only HT users showing a steeper aging trajectory than combination HT users (post-hoc; p=0.033; Figure 1). Duration of HT use and age at HT onset were associated with NODDI-ISOVF in estrogen-only compared to combination users (post-hoc; p=0.010 and p=0.013, respectively; Figure 2, top and middle panels); when adjusting for age at menopause, there was a significant association for NODDI-ISOVF and age (post-hoc; p=0.016; Figure 2, bottom panel). No significant associations with age were found across DTI, TDF and MAPMRI metrics.CONCLUSIONS
In this study of middle aged and older women, using advanced dMRI methods and accounting for differences in hormone therapy formulation, we showed that not all HT formulations exert a detectable neuroprotective effect. Hormone replacement therapy containing estrogen in combination with progestin may be associated with marginally more beneficial aging patterns (less pronounced white matter changes) than formulations containing estrogen alone. The recorded increases in the fractional volume of water molecules diffusing freely in the extracellular space, for women taking HT formulations containing estrogen alone, may suggest increased susceptibility to mechanisms of neuroinflammation or neuronal loss in this subgroup. These apparently unfavorable effects were independent of duration of hormone therapy, age at hormone therapy onset and age at menopause; however, these clinically relevant factors may modulate the more favorable effects observed with age on white matter in women following combination hormone replacement therapy.Acknowledgements
This research was supported by the National Institute of Aging (R56AG058854 and U01AG068057 to P.M.T. and R01AG059874 to N.J.), the National Institute of Biomedical Imaging and Bioengineering (P41EB015922 to P.M.T) and a grant from Biogen, Inc. (to P.M.T. and N.J.). UK Biobank research application #11559.References
1.Lange, A.-M. de et al. Cumulative estrogen exposure, APOE genotype, and women’s brain aging - a population-based neuroimaging study. bioRxiv 826123 (2019) doi:10.1101/826123.
2. De Lange, A.-M. G. et al. Women’s brain aging: effects of sex-hormone exposure, pregnancies, and genetic risk for Alzheimer’s disease. bioRxiv 1–22 (2020).
3. Song, Y. et al. The Effect of Estrogen Replacement Therapy on Alzheimer’s Disease and Parkinson’s Disease in Postmenopausal Women: A Meta-Analysis. Front. Neurosci. 14, 1–13 (2020).
4. Hoekzema, E. et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296 (2017).
5. Barth, C. et al. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Sci. Rep. 6, 1–9 (2016).
6. Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
7. Nir, T. M. et al. Fractional anisotropy derived from the diffusion tensor distribution function boosts power to detect Alzheimer’s disease deficits. Magn. Reson. Med. 78, 2322–2333 (2017).
8. Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–1016 (2012).
9. Fick, R. H. J., Wassermann, D., Caruyer, E. & Deriche, R. MAPL: Tissue microstructure estimation using Laplacian-regularized MAP-MRI and its application to HCP data. Neuroimage 134, 365–385 (2016).
10. Özarslan, E. et al. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage 78, 16–32 (2013).
11. Jahanshad, N. et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 81, 455–469 (2013).
12. Bycroft, C. et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv (2017) doi:10.1101/166298.
13. Townsend, P., Phillimore, P. & Beattie, A. Health and deprivation: inequality and the North. (Routledge, 1988).
14. Nobis, L. et al. Hippocampal volume across age: Nomograms derived from over 19,700 people in UK Biobank. NeuroImage Clin. 23, 101904 (2019).
15. Lv, J. et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. bioRxiv (2020) doi:10.1101/2020.01.17.911032.
16. Bethlehem, R. A. I., Seidlitz, J., Romero-Garcia, R., Dumas, G. & Lombardo, M. V. Normative age modelling of cortical thickness in autistic males. bioRxiv (2018) doi:10.1101/252593.
Figures