1705
Orthogonal diffusion tensor decomposition reveals age-related degeneration patterns in complex fibre architecture1Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
Diffusion tensor imaging (DTI) consistently detects increased mean diffusivity (MD) and decreased fractional anisotropy (FA) with age in single white matter (WM) bundles, but findings have been inconsistent in more complex fibre architecture. In this study, an orthogonal moment tensor decomposition yields clear degeneration patterns across complex fibre architecture, indicating that apparent challenges of DTI in these regions were due to the choice of tensor decomposition rather than the DTI model itself. This study therefore presents a revised view of DTI of aging WM and indicates how degeneration in complex fibre architecture can manifest in forms other than decreased FA.
Introduction
Diffusion tensor imaging (DTI) typically involves decomposing the tensor into its eigenvalues and computing mean diffusivity (MD) and fractional anisotropy (FA). DTI studies have consistently detected increased MD and decreased FA with advancing age in lateral regions of primarily single white matter (WM) fibre bundles, but findings have been inconsistent in medial regions of more complex fibre architecture. FA exhibits weak sensitivity to age in the corona radiata and internal capsule, and recent DTI studies with larger samples have found sporadic increases in FA with age, likely resulting from selective degeneration of secondary crossing fibres.1-2 The lack of more consistent detection of these increases in FA with age can be attributed to FA and MD not being mutually orthogonal,3 that is, increased MD with age masking increases in tensor anisotropy with age.Given that DTI remains more common for characterizing aging WM than advanced diffusion MRI models due to DTI’s simplicity, robustness and efficiency, it is critical to strive to maximize the information extracted from DTI across the entire WM, including regions of complex fibre architecture. Therefore, rather than stopping short at two non-orthogonal metrics of MD and FA, and rather than employing the eigenvalues directly, which do not provide a holistic representation of tensor shape, the present study employs a complete trivariate orthogonal decomposition of the tensor based on its eigenvalue moments.
Methods
Study ParticipantsAll subjects were participants of the UK Biobank.1 Subjects were excluded from our study if they reported a neurological, psychological or psychiatric disorder, neurological injury, or history of stroke. Subjects were then divided up into seven age categories of [46-50, 51-55, 56-60, 61-65, 66-70, 71-75, 76-80] years, and 50 male and 50 female subjects were randomly selected from each of the seven age categories. The final sample thus consisted of 700 subjects aged 46-80 (50% male, 50% female).
Diffusion MRI Data
Diffusion MRI data were acquired as part of the UK Biobank on a Siemens Skyra 3T system with 5 b=0, 50 b=1000s/mm2 and 50 b=2000s/mm2 volumes (100 distinct directions) at TR = 3.6s, TE=92ms, matrix size=104x104x72 with (2mm)3 resolution, 6/8 partial Fourier, 3x multislice acceleration and no in-plane acceleration. An additional 3 b=0 volumes were acquired with a reversed phase encoding. The data were corrected for eddy-current and susceptibility-related distortions via EDDY4 and TOPUP,5 respectively.
Data Processing
The diffusion tensor was computed voxelwise by fitting the diffusion MRI signal from the b=0 and b=1000s/mm2 shells to the DTI model. Three orthogonal DTI metrics {mean diffusivity (MD), norm of anisotropy (NA), mode of anisotropy (MO)} were computed based on the moments of the tensor’s eigenvalues {λ1,λ2,λ3} via3
MD = (λ1 + λ2 + λ3)/3
NA = √[(λ1-MD)2 + (λ2-MD)2 + (λ3-MD)2]
MO = 3√6 [(λ1-MD)(λ2-MD)(λ3-MD)]/NA3
To interpret age associations of these metrics in complex fibre architecture, BEDPOSTX was applied to the b=0, b=1000 and b=2000 s/mm2 shells to estimate the proportion of each crossing tract per voxel.6
Statistical Analysis
FSL’s Tract-Based Spatial Statistics (TBSS) was used to obtain a WM skeleton.7 For voxelwise analysis, a relationship of metric with age defined by p<0.05 with correction for multiple comparisons (cluster-wise controlling family-wise error rate with 5000 permutations) and controlling for gender as per FSL’s randomise.8 Age-related difference of metric per year was calculated using a general linear model as implemented in FreeSurfer.9
Results
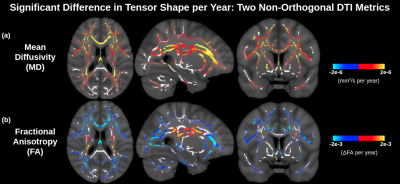
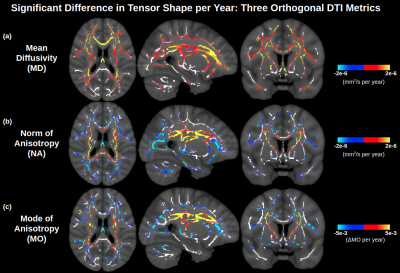
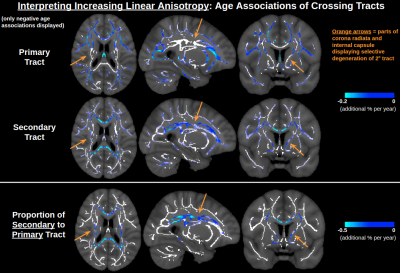
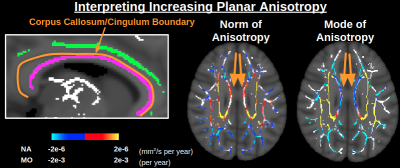
Figure 1 displays significant age associations of the conventional (non-orthogonal) DTI metrics MD and FA. FA is generally insensitive to age in medial regions, other than some sporadic positive age associations of FA. In Figure 2, it is seen that the orthogonal decomposition yields clear and consistent positive age associations of NA throughout these medial regions. In conjunction with MO, the orthogonal decomposition reveals increased linear anisotropy with advancing age in the corona radiata, internal capsule and corpus callosum splenium, and increasing planar anisotropy with advancing age in the corpus callosum body. When comparing with BEDPOSTX fibre-fraction outputs, increasing linear anisotropy can be interpreted as selective degeneration of secondary crossing fibres (anterior-posterior association fibres) while the principal fibres (superior-inferior projection fibres) remain relatively intact (Figure 3), and increasing planar anisotropy can be interpreted as degeneration of callosal fibres alongside decreased partial-voluming with cingulum fibres (Figure 4).Discussion and Conclusion
Use of an orthogonal moment tensor decomposition revealed positive age associations of anisotropy in regions of complex fibre architecture that exhibited limited age associations of FA. Specifically, while FA demonstrates low sensitivity to age in the corona radiata, internal capsule and posterior corpus callosum, NA and MO are consistently sensitive to age throughout these regions, indicating that the previous challenges of DTI in these regions were due to the choice of decomposition rather than the DTI model itself. Therefore, this study demonstrates that if an advanced diffusion MRI technique is not feasible, the conventional DTI model can still be used to gain insight into crossing fibre regions by using the orthogonal moment decomposition, bearing in mind that the complex fibre architecture of WM tissue means that degeneration does not always manifest itself as decreases in tensor anisotropy.Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 40922. We thank CIHR, NSERC and NIH for financial support.References
1. Miller KL et al (2016) Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19:1523-1536.
2. de Groot M et al (2016) White Matter Degeneration with Aging: Longitudinal Diffusion MR Imaging Analysis. Radiology 279:532-541.
3. Ennis DB and Kindlmann G (2006) Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med 55:136-146.
4. Andersson JLR and Sotiropoulus SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063-1078.
5. Andersson JLR, Skare S and Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20:870-888.
6. Jbabdi S et al (2012) Model-based analysis of multishell diffusion MR data for tractography: how to get over fitting problems. Magn Reson Med 68:1846-1855.
7. Smith SM et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487-1505.
8. Winkler AM et al (2014) Permutation inference for the general linear model. Neuroimage 92:381-397.
9. Fischl B (2012) FreeSurfer. Neuroimage 62:774-781.
Figures