1704
Using diffusional kurtosis imaging to capture white matter tissue complexity in aging: Does the choice of software package affect results?
Hiba Taha1,2, Jordan A. Chad2,3, and J. Jean Chen2,3
1Human Biology, University of Toronto, Toronto, ON, Canada, 2Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 3Medical Biophysics, University of Toronto, Toronto, ON, Canada
1Human Biology, University of Toronto, Toronto, ON, Canada, 2Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 3Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Studies in white matter (WM) aging have previously used diffusional kurtosis imaging (DKI), however it remains unclear if results have been affected by limited sample sizes or the choice of software package. In this study, we show positive age associations of diffusivity and negative age associations of kurtosis throughout WM tracts using two different DKI packages on a large sample of 700 adults. Unique regional specific changes were observed differentially using these two tools, suggesting that the choice of software package can influence the study of aging.
Introduction
Previous studies in healthy aging using diffusion tensor imaging (DTI) have found age-associated decreases in fractional anisotropy (FA) and increases in radial diffusivity (RD) and mean diffusivity (MD), suggesting an overall decrease in WM integrity1-3. However, conventional DTI fails to take into account non-Gaussian diffusion in the WM that may arise due to cellular membranes, multiple tissue compartments or metabolic debris, leading to limited specificity to microstructure. Diffusion kurtosis imaging (DKI) allows for the measurement of non-Gaussian diffusion, but very few studies in aging have reported the use of DKI. In these studies, reductions in kurtosis metrics axial kurtosis (AK), mean kurtosis (MK) and radial kurtosis (RK) with age were observed, suggesting a global decrease in WM complexity with age 4-9. While it is imperative to replicate the findings of these studies on a larger sample, an additional complication is that many DKI software packages have been developed in recent years and it is currently unclear which package would be an optimal choice. In addition to the standard DKI toolbox implemented in Matlab,10 the recently released DKI tool available through DIPY’s diffusion MRI toolbox11 has yet to be systematically investigated. In the current study, two main DKI-fitting methods are compared in a large cross-sectional sample to address two main questions: (1) how do DKI and DTI differentially reflect age-associated changes in WM? and, (2) does the kurtosis-fitting package implemented affect the derived results?Methods
Study ParticipantsMRI data acquired from a sample of 700 individuals (50% male) aged 46 to 80 years from the UKBiobank Initiative (Application 40922; ukbiobank.co.uk) were used for this study12. Subjects reporting neurological, psychological or psychiatric disorders, neurological injury or history of stroke were excluded from this study.
Data Acquisition
Diffusion MRI data were acquired using a Siemens Skyra 3T system with 5 b=0, 50 b=1000s/mm2, and 50 b=2000s/mm2 across 100 distinct directions at TR = 3.6s, TE = 92ms, matrix size = 104x104x72 with 8mm3 resolution, 6/8 partial Fourier, 3x multislice acceleration and no in-plane acceleration. Additional 3 b=0 volumes were attained with reversed phase encoding. Data was corrected for eddy currents and susceptibility-related distortions using EDDY and TOPUP.
Data Processing
Diffusion tensor and kurtosis models were fitted using two tools: The standard approach implemented in the Matlab Toolbox,10 and a recent toolbox developed as part of DIPY 11. DTI metrics fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD), radial diffusivity (RD) and DKI metrics axial kurtosis (AK), mean kurtosis (MK) and radial kurtosis (RK) were acquired from both tools. Additional metrics kurtosis of fractional anisotropy (KFA) and mean kurtosis tensor (MKT) were acquired using the DIPY tool.
Statistical Analysis
To assess age-related associations of these metrics, whole-brain voxel-based statistical analysis was conducted using Tract-Based Spatial Statistics (TBSS) and FSL’s randomise tool. Analysis was performed using Threshold-Free Cluster Enhancement (TFCE) with 500 permutations. The FA skeleton is presented with a threshold value of 0.2 and all age-associated metric changes using a significance value of p < 0.05.
Results
A general trend of positive age associations in diffusivity depicted by MD and RD were observed throughout the WM skeleton, along with negative age associations in FA and kurtosis depicted by AK, MK, RK using both tools. In addition, positive age associations of FA were captured in the left and right corticospinal tract (CST) regions using the DKI Matlab toolbox (and not with DIPY), which overlapped with positive age associations of AD (Figure 1). This age-related increase in FA is consistent with the literature 13, 14. Moreover, age associations of AD were regionally specific such that positive age associations were observed in the anterior and superior regions, while negative age associations were restricted to posterior and inferior regions (Figure 1). Kurtosis metrics generally exhibited negative associations with age, with additional metrics such as KFA available through the DIPY tool only (Figure 2). Positive age associations of AK were observed using the DIPY tool in parts of the corpus callosum and forceps minor, very little negative associations were observed in AD, and no positive age associations were observed in FA (Figure 3).Discussion and Conclusion
Results derived from both tools indicate an age-associated global increase in diffusivity with decrease in kurtosis, consistent with previous findings of declining tissue microstructural complexity and diffusional heterogeneity in aging. DTI and DKI results both reveal patterns of change consistent with the anterior-posterior gradient of aging15. However, the DIPY tool yielded no positive age-associations of FA, a sign of selective degeneration of the fibres resulting in more anisotropy and increased diffusivity along the principal tract14. DIPY also yielded few negative age-associations of AD, and unexpected positive age-associations of AK, inconsistent with previous findings. Nonetheless, the DIPY tool appears more sensitive in capturing significant diffusion and kurtosis changes. It is unclear whether these differences are due to excessive smoothing and less constraints in DIPY. Future work will include more rigorously comparing these findings with conventional DTI age effects.Acknowledgements
We thank the Canadian Institutes for Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support.References
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating Imaging Indices of White Matter Integrity and Volume in Healthy Older Adults. Cerebral Cortex. 2007;18(2):433–442.
- Hsu J-L, Leemans A, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Chen W-H. Gender differences and age-related white matter changes of the human brain: A diffusion tensor imaging study. NeuroImage. 2008;39(2):566–577.
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping. 2009;31(3):378–390.
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. International Journal of Geriatric Psychiatry. 2009;24(2):109–117.
- Coutu JP, Chen JJ, Rosas HD, Salat DH. Non-Gaussian water diffusion in aging white matter. Neurobiology of Aging. 2014; 35; 1412-1421.
- Lätt J, Nilsson M, Wirestam R, Ståhlberg F, Karlsson N, Johansson M, Sundgren PC, Westen DV. Regional values of diffusional kurtosis estimates in the healthy brain. Journal of Magnetic Resonance Imaging. 2012;37(3):610–618.
- Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Martino AD, Ferris SH, Helpern JA. Age-related non-Gaussian diffusion patterns in the prefrontal brain. Journal of Magnetic Resonance Imaging. 2008;28(6):1345–1350.
- Das SK, Wang JL, Bing L, Bhetuwal A, Yang HF. Regional Values of Diffusional Kurtosis Estimates in the Healthy Brain during Normal Aging. Clinical Neuroradiology. 2016;27(3):283–298.
- Beck D, de Lang AG, Maximov II, Richard G, Andreassen OA, Nordvik JE, Westlye LT. White matter microstructure across the adult lifespan: A mixed longitudinal and cross-sectional study using advanced diffusion models and brian-age prediction. NeuroImage. 2021; 224:117441.
- Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. NeuroImage. 2013; 81:335-346.
- Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, Nimmo-Smith I, Dipy Contributors. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014; 8:8.
- UK Biobank online [Internet]. C2019. Biobank: Improving the health of future generations. England and Wales. [cited May 25 2020]. https://www.ukbiobank.ac.uk/
- Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, Bartsch AJ, Jbabdi S, Sotiropoulous SN, Andersson JLR, Griffanti L, Douaud G, Okell TW, Weale P, Dragonu I, Garratt S, Hudson S, Collins R, Jenkinson M, Matthews PM, Smith SM. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016; 19:1523-1536.
- de Groot M, Cremers LGM, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Niessen WJ, Vernooij MW. White Matter Degeneration with Aging: Longitudinal Diffusion MR Imaging Analysis. Radiology. 2016; 279:532-541.
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: a diffusion-tensor analysis. Arch Gerontol Geriatr. 2008; 47(1):129-38
Figures
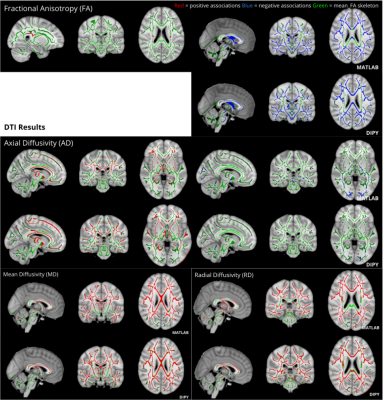
Figure 1. Matlab Toolbox and DIPY derived age-associated significant diffusion metric changes (p<0.05) across the WM skeleton presented in radiological orientation. Diffusion metrics mostly increased with age, with the exception of FA which showed reductions throughout the WM skeleton, and AD which showed specific reductions in posterior regions. FA increases in the right and left corticospinal tracts were also observed using Matlab only.
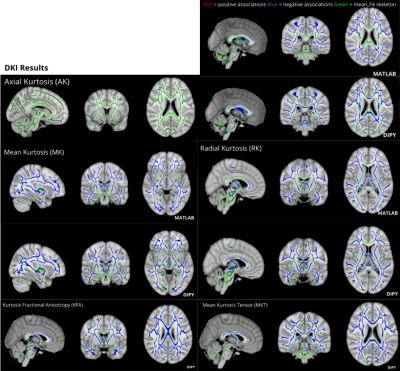
Figure 2. Matlab Toolbox and DIPY derived age-associated significant kurtosis metric changes (p<0.05) across the WM skeleton presented in radiological orientation. Kurtosis metrics mostly decreased with age, with the exception of AK increases captured by DIPY only in the corpus callosum. Additional kurtosis metrics KFA and MKT were available for computation using DIPY only, showing age-associated decreases.
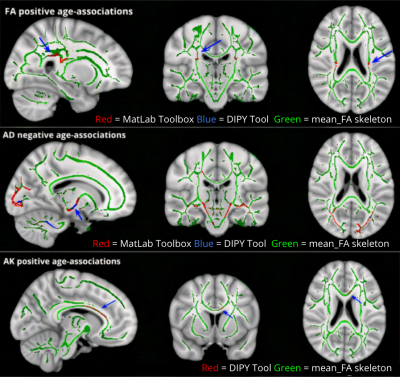
Figure 3. DIPY derived unexpected significant diffusion and kurtosis age-associated findings (p<0.05) across the WM skeleton. No significant positive associations of FA and very little negative associations were observed of AD with age were observed using DIPY. Positive age-associations of AK were observed using DIPY only, depicted in red, specifically along the corpus callosum and forceps minor.