1701
Profiling diffusion at the grey matter-white matter interface (GWI) to reveal unique microstructural features: proof of concept in aging1Radiology, Albert Einstein College of Medicine, Bronx, NY, United States
Synopsis
Microstructure differs greatly between cortical gray matter and adjacent white matter and this point of transition, the gray-white matter interface, is a predilection site for pathologies, including traumatic brain injury, small vessel vasculitis and microembolic ischemic injury. The interface region presents challenges to both voxel-wise and region of interest (ROI) analyses, because small registration or ROI placement errors may lead to large errors in extracted diffusion metrics. We propose an approach to assess alteration of the sharpness of the gray-white matter interface and illustrate its potential utility through the detection of age-related decline of the sharpness of this transition.
Introduction
Alternations in cerebral structure occur as a result of normal brain development and aging as well as a sequela of injury to the brain and manifest on MRI images with a variety of contrast weightings. Standard approaches to detect and quantify such alterations are focused on volume and thickness of cortical gray matter on the one hand and microstructural alteration in deep white matter tracts on the other. However, the interface of gray and white matter is an area that may be a predilection site for injury, as is for example known to be the case in brain trauma,1,2 vasculitis and embolic ischemic injury. Despite its importance, quantitative analysis of the interface region presents challenges to both voxel-wise and region of interest (ROI) analyses, because small registration or ROI placement errors may lead to large errors in extracted diffusion metrics.We developed an approach to assess alteration of the sharpness of the gray matter-white matter interface (GWI) and for this proof of concept illustrate it on the example of fractional anisotropy (FA) derived from diffusion tensor imaging (DTI) across wide age span. Sharpness of the interface is defined as the largest slope of the whole brain averaged FA profile across anatomically defined gray matter-white matter boundary. We expect decline of the sharpness of this transition in normal aging because of associated microstructural changes such as demyelination and neuronal loss. In applications other than aging, gray-white matter interface can be examined over smaller more relevant and specific brain regions.
Methods
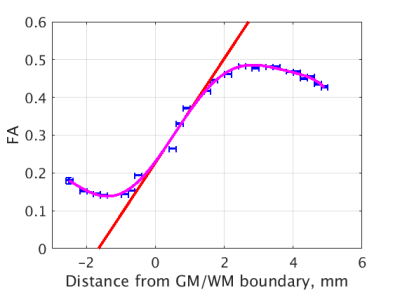
This study was approved by IRB and includes 96 datasets (age range: 20-63, 50 females) obtained as part of ongoing Einstein Lifespan Study (ELS) and 44 datasets (age range: 71-88, 29 females) obtained as part of ongoing Einstein Aging Study (EAS). Images were reviewed by an experienced neuroradiologist and determined to be free of visible structural abnormalities. Imaging was performed using a 3.0T Philips Achieva TX scanner (Philips Medical Systems, Best, The Netherlands) utilizing its 32-channel head coil with the following protocol. T1W: TR/TE/TI = 9.9/4.6/900 msec, flip angle 8deg, 1mm3 isotropic resolution, 128x116x220 matrix; DTI: TR/TE = 10,000/65msec, 32 diffusion directions, b-value = 800 sec/mm2, 2mm3 isotropic resolution, 240x188x70 matrix; and field map to remove EPI distortions in DTI and small distortions in T1W: TR/TE = 20/2.4 msec, delta TE=2.3msec, flip angle 20deg, 4mm3 isotropic resolution, 64x64x50 matrix. DTI data were eddy corrected3 and registered to T1W. For each registration, MARLINA4 selected the best of the 26 candidates produced with FLIRT of FSL3. All brain extractions and registrations were also visually inspected.Gray-white matter boundary was delineated over T1W images using ASEG module of FreeSurfer6 version 6.0. The interface region was limited to 5mm around it. The FA profile of cortical gray matter-white matter transition for each individual subject was constructed by computing shortest Euclidian distance5 from each voxel to the gray-white matter boundary and averaging all FA values at a given distance. These average FA values were weighted by their standard deviation to fit a 7th degree polynomial in order to smooth the profile and determine its maximum slope in the vicinity of the gray-white matter boundary (see Figure 1).
Results
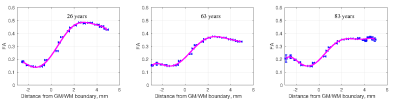
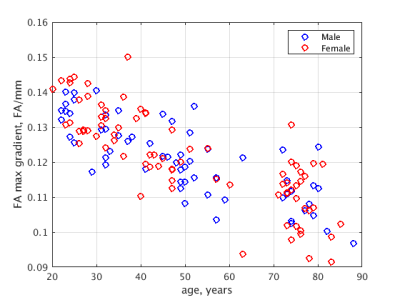
Example profile plots (Figure 2) show age-dependence of the GWI transition of FA for young, middle-aged and elderly participants. Maximum slope of FA across the GWI declines with advancing age, consistent with known age-related effects on brain structure. Figure 3 demonstrates the phenomenon does not diverge by sex.Discussion
In whole-brain applications such as presented, no additional processing steps are needed because gray matter completely encloses the white. For localized GWI analysis to ensure the shortest distance is in fact measured perpendicular to the interface voxels within the white matter where the shortest distance to its complement differs from the shortest distance to the gray matter are to be discarded. Similarly should be discarded voxels within the gray matter.Spatial location at which the maximum slope is realized (the inflection point) might be considered as defining gray-white boundary based on microstructural tissue characteristics. Its displacement from the anatomical boundary might also be region dependent. At the same time, FA profile in its vicinity is close to linear (Figures 1,2), making exact localization of the inflection point ill-defined. We therefore focused analysis of GWI on the slope.
Conclusion
We proposed and implemented a novel method to characterize microstructural features that alter the expected sharpness of the GWI. Our recapitulation of age-related trends similar to other DTI studies of aging demonstrates the potential for this approach to detect very subtle alternations that may not be identified with existing techniques and can be extended to provide assessment of the GWI over smaller regions. The technique holds particular promise to advance understanding the microstructural features of disorders that impact the GWI.Acknowledgements
Support for this research was provided in part by the National Institute on Aging, grant 5P01AG003949-34.References
1. Schaefer PW, Huisman TA, Sorensen AG, Gonzalez RG, Schwamm LH. Diffusion-weighted MR imaging in closed head injury: high correlation with initial glasgow coma scale score and score on modified Rankin scale at discharge. Radiology. 2004;233(1):58-66. Epub 2004/08/12. doi: 10.1148/radiol.2323031173. PubMed PMID: 15304663.
2. Stojanovski S, Nazeri A, Lepage C, Ameis S, Voineskos AN, Wheeler AL. Microstructural abnormalities in deep and superficial white matter in youths with mild traumatic brain injury. Neuroimage Clin. 2019;24:102102. Epub 2019/12/05. doi: 10.1016/j.nicl.2019.102102. PubMed PMID: 31795058; PMCID: PMC6889799.
3. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782-90.
4. Fleysher R, Fleysher L, Suri A, Zimmerman M, Jenkinson M, Branch CA, Lipton ML. Artificial Observer and Cost Function for Image Registration, MARLINA: Mean Absolute Regional LINear correlation Algorithm. Proceedings of 27th ISMRM 2019; 2706
5. Mishchenko Y. A fast algorithm for computation of discrete Euclidean distance transform in three or more dimensions on vector processing architectures. Signal, Image and Video Processing. 2013;9(1):19-27. doi: 10.1007/s11760-012-0419-9.
6. Fischl B., Salat D. H., Busa E., et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002; 33: 341.
Figures