1699
Age and Sex Effects on Brain White Matter Microstructure assessed with Advanced Single- and Multi-Shell Diffusion MRI Metrics1University of Southern California, Marina del Rey, CA, United States
Synopsis
Characterizing the brain’s white matter microstructure is crucial for improving our understanding of healthy and diseased aging. Here we examined the ability of both traditional diffusion methods (diffusion tensor imaging) and advanced diffusion methods (tensor distribution function, neurite orientation dispersion and density imaging, mean apparent propagator MRI) to capture age and sex effects on white matter microstructure in a large sample of aging adults (15,628 UK Biobank participants; age range 45-80 years). Advanced diffusion models exhibited the greatest sensitivity to participant age and sex, suggesting that future aging studies may benefit from using advanced diffusion approaches.
Introduction
White matter degradation in aging has been linked to age-related cognitive decline and neurodegenerative conditions such as Alzheimer’s disease.1,2 Most neuroimaging studies of white matter microstructure during aging have so far examined diffusion-weighted MRI (dMRI) data fit with the diffusion tensor imaging (DTI) model.3 However, more recently developed dMRI models – such as the tensor distribution function (TDF), neurite orientation dispersion and density imaging (NODDI), and mean apparent propagator MRI (MAPMRI) – may provide more refined representations of underlying white matter properties.4-10 Here we investigated the ability of both traditional diffusion methods (DTI) and advanced diffusion methods (TDF, NODDI, MAPMRI) to capture age and sex differences in white matter microstructure during aging. We also created sex-stratified centile reference curves for each dMRI model to provide normative models of white matter aging.11-13Methods
Data Acquisition and ProcessingThe UK Biobank is a publicly available dataset of community-based middle-aged and older adults residing in the United Kingdom.14 Here we analyzed cross-sectional brain dMRI data from a total of 15,628 UK Biobank participants (age: 45-80 years; 47.6% male, 52.4% female).15 For single-shell dMRI data (b=1000 s/mm2, 50 gradients), we used the traditional model, DTI, and the advanced model, TDF, where the former fits a single-tensor model and the latter fits a continuous distribution of tensors to model multiple underlying fiber populations.3,6 Metrics derived from DTI included fractional anisotropy (DTI-FA), mean diffusivity (DTI-MD), axial diffusivity (DTI-AD), and radial diffusivity (DTI-RD). TDF was used to derive an advanced measure of fractional anisotropy (TDF-FA). For multi-shell dMRI data (b=1000 and 2000 s/mm2, 100 gradients total), the advanced models NODDI and Laplacian-regularized MAPMRI were fit using the AMICO tool and DIPY, respectively.9,10,16 NODDI separately models intra-cellular, extra-cellular, and isotropic water components, providing microstructure metrics that may be more closely linked to specific aspects of the cellular environment than DTI or TDF.8 MAPMRI estimates the diffusion patterns of water molecules without making any assumptions about the underlying tissue architecture.9,10,17 The following white matter indices were calculated using NODDI: orientation dispersion (OD), intra-cellular volume fraction (ICVF), and isotropic volume fraction (ISOVF). Measures derived from MAPMRI included return-to-origin probability (RTOP), return-to-axis probability (RTAP), and return-to-plane probability (RTPP). Diffusion-weighted MRI metrics were projected to a standard white matter skeleton using publicly available ENIGMA protocols based on FSL’s TBSS, and mean whole-skeleton diffusivity values were extracted for each metric.18,19
Statistical Analyses
We investigated the effects of age, sex, and their interaction on each dMRI metric. For our primary analyses, fractional polynomials were used to find the best-fitting model for age for each metric by testing one- and two-term curvilinear models using the following possible powers: -2, -1, -0.5, 0, 0.5, 1, 2 and 3, where x0 corresponds to ln(x).20-22 All analyses included the following covariates:23 educational attainment (operationalized as “college” or “no college”), socioeconomic status (quantified using the Townsend Deprivation Index24), waist-hip ratio, and population structure (measured using the first 4 principal components obtained from the UK Biobank’s genetic ancestry analyses25.) Effect sizes were calculated as the difference in variance explained by age, sex, and the age by sex interaction separately. For instance, the effect size for age was computed as the difference in variance (change in R2) between two models: one which included the age terms in addition to sex and nuisance covariates, and one which only included sex and nuisance covariates.17 To confirm our results were robust to statistical model, we repeated analyses by modeling age as a binary variable with two participant groups: > 60 years old and < 60 years old.21,22 Lastly, sex-stratified normative centile reference curves11-13 were created for each diffusivity metric by using quantile regression to model age continuously with fractional polynomials.
Results
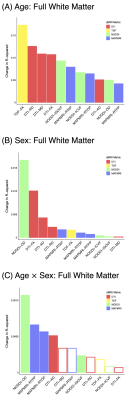
Age robustly impacted all dMRI metrics. The advanced single-shell model, TDF, was most sensitive to age as indicated by effect size, followed by the traditional single-shell method, DTI (Figure 1A). Follow-up exploratory analyses categorizing participants into two age groups (> 60 years and < 60 years) yielded comparable findings (Figure 2A).Participant sex was likewise significantly associated with nearly all dMRI measures (Figure 1B). Sex differences in white matter microstructure were detected most sensitively by the multi-shell model, NODDI, followed by the single-shell model, DTI. Supplementary analyses splitting subjects into those younger or those older than 60 years old provided similar results (Figure 2B).
The impact of age also significantly depended on sex for multiple dMRI indices (Figure 1C). The multi-shell model, NODDI, was the most sensitive to sex differences in aging, followed by the multi-shell model, MAPMRI. Additional analyses treating age as a binary variable (participants > 60 years vs. < participants 60 years) likewise indicated significant age by sex interactions, with NODDI again exhibiting the largest effect size (Figure 2C).
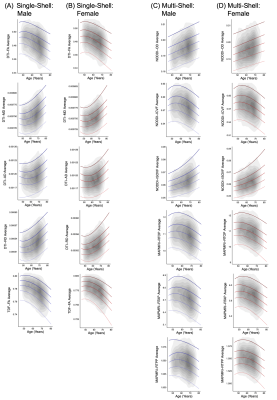
Normative centile reference curves for each dMRI metric are presented for male and female participants separately in Figure 3.
Conclusion
Advanced dMRI models exhibited the greatest sensitivity to age- and sex-associated white matter variability among aging adults. Future aging research examining the impact of age and participant sex may benefit from including advanced dMRI measures.Acknowledgements
This research was supported by the National Institute of Aging (award numbers R56AG058854 and U01AG068057 to P.M.T., and R01AG059874 to N.J.), the National Institute of Biomedical Imaging and Bioengineering (award number P41EB015922 to P.M.T.), the National Institute of Mental Health (award number F32MH122057 to K.E.L.), and a grant from Biogen, Inc. (to P.M.T. and N.J.). This research was conducted using the UK Biobank resource under Application 11559.References
1. Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187-205.
2. Pievani M, Filippini N, van den Heuvel MP, Cappa SF, Frisoni GB. Brain connectivity in neurodegenerative diseases--from phenotype to proteinopathy. Nat Rev Neurol. 2014;10(11):620-633.
3. Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936-952.
4. Leow AD, Zhu S, Zhan L, et al. The tensor distribution function. Magn Reson Med. 2009;61(1):205-214.
5. Zhan L, Leow AD, Zhu S, et al. A novel measure of fractional anisotropy based on the tensor distribution function. Med Image Comput Comput Assist Interv. 2009;12(Pt 1):845-852.
6. Nir TM, Jahanshad N, Villalon-Reina JE, et al. Fractional anisotropy derived from the diffusion tensor distribution function boosts power to detect Alzheimer's disease deficits. Magn Reson Med. 2017;78(6):2322-2333.
7. Villalon-Reina JE, Ching CRK, Kothapalli D, D. S, Nir T. Alternative diffusion anisotropy measures for the investigation of white matter alterations in 22q11.2 deletion syndrome. Proc SPIE 10975. 2018:1-13.
8. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016.
9. Ozarslan E, Koay CG, Shepherd TM, et al. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage. 2013;78:16-32.
10. Fick RHJ, Wassermann D, Caruyer E, Deriche R. MAPL: Tissue microstructure estimation using Laplacian-regularized MAP-MRI and its application to HCP data. Neuroimage. 2016;134:365-385.
11. Nobis L, Manohar SG, Smith SM, et al. Hippocampal volume across age: Nomograms derived from over 19,700 people in UK Biobank. Neuroimage Clin. 2019;23:101904. 12. Bethlehem RAI, Seidlitz J, Romero-Garcia R, Dumas G, Lombardo MV. Normative age modelling of cortical thickness in autistic males. bioRxiv. 2019:1-23.
13. Lv J, Di Biase M, Cash RFH, et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. 2020:1-24.
14. Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523-1536.
15. Alfaro-Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400-424.
16. Daducci A, Canales-Rodriguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage. 2015;105:32-44.
17. Pines AR, Cieslak M, Larsen B, et al. Leveraging multi-shell diffusion for studies of brain development in youth and young adulthood. Dev Cogn Neurosci. 2020;43:100788.
18. Jahanshad N, Kochunov PV, Sprooten E, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455-469.
19. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505. 20. Royston P, Altman D. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Applied Statistics. 1994;43(3):429-467.
21. Dima D, Papachristou E, Modabbernia A, et al. Subcortical Volume Trajectories across the Lifespan: Data from 18,605 healthy individuals aged 3-90 years. bioRxiv. 2020:2020.2005.2005.079475.
22. Frangou S, Modabbernia A, Doucet GE, et al. Cortical Thickness Trajectories across the Lifespan: Data from 17,075 healthy individuals aged 3-90 years. bioRxiv. 2020:2020.2005.2005.077834.
23. Salminen LE, Wilcox RR, Zhu AH, et al. Altered Cortical Brain Structure and Increased Risk for Disease Seen Decades After Perinatal Exposure to Maternal Smoking: A Study of 9000 Adults in the UK Biobank. Cereb Cortex. 2019;29(12):5217-5233.
24. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. Routledge; 1988.
25. Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv. 2017:166298.
Figures