1697
An Automated Processing Pipeline for Diffusion MRI in the Baby Connectome Project1Biomedical Research Imaging Center, University of North Carolina, Chapel Hill, Chapel Hill, NC, United States
Synopsis
Processing baby diffusion MRI (dMRI) data is challenging due to the low and spatially-varying diffusion anisotropy, rendering standard analysis techniques developed for adult data inapplicable [1,2]. Here, we present a fully-automated processing pipeline for baby dMRI, tailored particularly to the data collected in the Baby Connectome Project (BCP).
Synopsis
Processing baby diffusion MRI (dMRI) data is challenging due to the low and spatially-varying diffusion anisotropy, rendering standard analysis techniques developed for adult data inapplicable [1,2]. Here, we present a fully-automated processing pipeline for baby dMRI, tailored particularly to the data collected in the Baby Connectome Project (BCP).Introduction
The human brain is arguably the most complex system in biology and yet its macroscopic layout is nearly complete by the time of term birth. The infant brain develops rapidly during the first years of life, posing significant challenges to precise quantification of rapid dynamic changes that occur during this critical period of brain development.The increasing availability of longitudinal baby MRI data, such as those acquired through the Baby Connectome Project (BCP) [3], affords unprecedented opportunity for precise charting of early brain developmental trajectories in order to understand normative and aberrant growth.
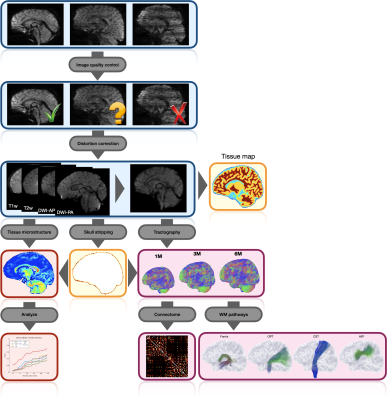
We describe here an automated processing pipeline for baby dMRI, consisting of (i) image quality control (IQC) for distinguishing between good and poor quality data, (ii) anatomy-guided correction for eddy-current and susceptibility-induced distortions, (iii) tissue microstructural analysis, and (iv) reconstruction of white matter (WM) pathways.
Methods
DMRI acquisitionIn the BCP [3], diffusion-weighted (DW) images were acquired using Siemens 3T Magnetom Prisma MRI scanners with a $$$140 \times 140$$$ imaging matrix, $$$1.5 \times 1.5 \times 1.5 \,mm^3$$$ resolution, $$$TE=88 \, ms$$$, $$$TR=2,365 \, ms$$$, 32-channel receiver coil, and $$$b=500, 1000, 1500, 2000, 2500, 3000 \, s/mm^2$$$, covered by a total of 144 noncollinear gradient directions for either posterior-anterior or anterior-posterior phase-encoding directions (PEDs). 6 non-DW images were collected for each PED.
Image quality control (IQC)
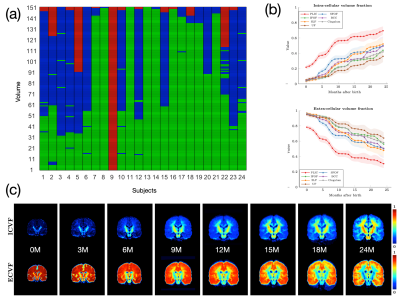
The DW images are annotated as "pass" (no/minor artifacts), "questionable" (moderate artifacts), or "fail" (heavy artifacts). The IQC framework utilizes a nonlocal residual network [4,5] to first examine the quality of each imaging slice. Slice-level features are then agglomerated for volume-level quality assessment, which is in turn used to arrive at the assessment outcome at the subject-level. Subjects annotated as "fail" are excluded from subsequent processing and analysis.
Distortion correction
The DW images associated with each PED are first corrected for eddy-current distortion by affine registration followed by coarse deformable registration to their corresponding non-DW image using ANTs [6]. To correct for susceptibility-induced distortion, we first compute the spherical mean images (SMIs) of the eddy-current corrected DW images, and jointly register the SMIs of all shells and the non-DW images to the corresponding aligned T1-weighted (T1w) and T2-weighted (T2w) images. The corrected DW images for both PEDs are obtained by one-time warping of the original DW images via composed deformation fields. Finally, the warped DW images for opposite PEDs are combined via the signal harmonic mean [7,8].
Tissue microstructure
A recent method called spherical mean spectrum imaging (SMSI) [9] is used to quantify tissue microarchitecture. SMSI allows a wide variety of features, such as intracellular volume fraction (ICVF), extracellular volume fraction (ECVF), free-water volume fraction, intra-soma volume fraction [10], anisotropy, and orientation coherence index, to be computed for comprehensive microstructural analysis. SMSI characterizes the whole fine- to coarse-scale diffusion spectrum and is hence well suited for capturing dynamic microstructural changes in the baby brain. SMSI has been recently demonstrated to show greater sensitivity to developmental changes via a multivariate framework [11], revealing distinct longitudinal patterns of both cortical and subcortical areas.
White matter pathways
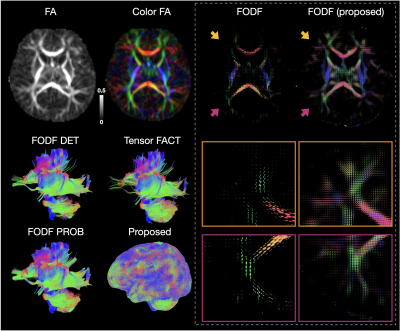
WM tractography is generated by fiber tracking using super-resolution asymmetry spectrum imaging (ASI) [1,12,13]. ASI fits a mixture of asymmetric fiber orientation distribution function (FODF) to the diffusion signal [1,13]. WM tractograms are then generated by successively following local directions determined from the FODFs [12]. ASI-based tractography mitigates the gyral bias problem [1,12] common in existing tractography algorithms and improves cortico-cortical connectivity in the baby brain. We employ TractDL [14] for fast and robust identification of fiber bundles of interest from a large number of streamlines. More than 160 anatomical WM pathways are identified consistently [15].
Results
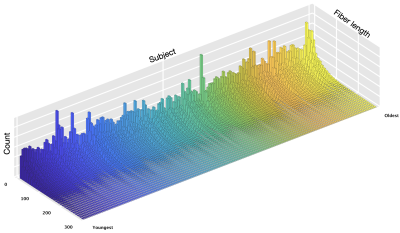
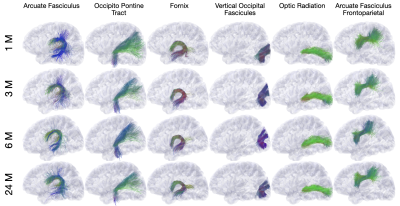
Fig. 1 shows an overview of our pipeline. Fig. 2 shows a visualization of the quality ratings for the volumes across subjects. Fig. 3 shows the trajectories of the ICVF and ECVF from birth to 2 years of age. Fig. 4 indicates that ASI improves FODF estimation compared with multi-tissue constrained spherical deconvolution [16]. Fig. 5 demonstrates that the fiber length distributions are highly consistent across 190 scans from 0 to 5 years of age. Fig. 6 indicates that six challenging white matter pathways can be reconstructed consistently across time using ASI-based tractography.Conclusion
We have introduced an automated pipeline for robust processing of baby dMRI data, involving image quality control, distortion correction, microstructural analysis, and mapping of white matter pathways.Acknowledgements
This work was supported in part by NIH grants (NS093842 and MH110274) and the efforts of the UNC/UMN Baby Connectome Project Consortium.
Y. Wu, S. Ahmad, K.M. Huynh, and S. Liu contributed equally to this work.
References
[1]. Wu Y, Lin W, Shen D, Yap PT. Asymmetry Spectrum Imaging for Baby Diffusion Tractography. Inf Process Med Imaging. 2019.
[2]. Huynh KM, Chen G, Wu Y, Shen D, Yap P-T. Multi-Site Harmonization of Diffusion MRI Data via Method of Moments. IEEE Trans Med Imaging. 2019;38: 1599–1609.
[3]. Howell BR, Styner MA, Gao W, Yap P-T, Wang L, Baluyot K, et al. The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. Neuroimage. 2019;185: 891–905.
[4]. Liu S, Thung K-H, Lin W, Shen D, Yap P-T. Hierarchical Nonlocal Residual Networks for Image Quality Assessment of Pediatric Diffusion MRI With Limited and Noisy Annotations. IEEE Trans Med Imaging. 2020;39: 3691–3702.
[5]. Liu S, Thung K-H, Lin W, Yap P-T, Shen D. Real-Time Quality Assessment of Pediatric MRI via Semi-Supervised Deep Nonlocal Residual Neural Networks. IEEE Trans Image Process. 2020.
[6]. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12: 26–41.
[7]. Ahmad S, Wu Z, Li G, Wang L, Lin W, Yap P-T, et al. Surface-constrained volumetric registration for the early developing brain. Med Image Anal. 2019;58: 101540.
[8]. Ahmad S, Wu Y, Huynh KM, Thung K-H, Lin W, Shen D, et al. Fast Correction of Eddy-Current and Susceptibility-Induced Distortions Using Rotation-Invariant Contrasts. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020. Springer International Publishing; 2020. pp. 34–43.
[9]. Huynh KM, Xu T, Wu Y, Wang X, Chen G, Wu H, et al. Probing Tissue Microarchitecture of the Baby Brain via Spherical Mean Spectrum Imaging. IEEE Trans Med Imaging. 2020;PP: 1–1.
[10]. Huynh KM, Wu Y, Thung K-H, Ahmad S, Taylor HP IV, Shen D, et al. Characterizing Intra-soma Diffusion with Spherical Mean Spectrum Imaging. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020. Springer International Publishing; 2020. pp. 354–363.
[11]. Huynh KM, Wu Y, Thung K-H, Ahmad S, Taylor HP IV, Lin W. Multivariate Quantification of Brain Development During the First Two Years of Life.
[12]. Wu Y, Hong Y, Feng Y, Shen D, Yap P-T. Mitigating gyral bias in cortical tractography via asymmetric fiber orientation distributions. Med Image Anal. 2020;59: 101543.
[13]. Wu Y, Hong Y, Ahmad S, Chang W-T, Lin W, Shen D, et al. Globally Optimized Super-Resolution of Diffusion MRI Data via Fiber Continuity. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020. Springer International Publishing; 2020. pp. 260–269.
[14]. Wu Y, Hong Y, Ahmad S, Lin W, Shen D, Yap P-T. Tract Dictionary Learning for Fast and Robust Recognition of Fiber Bundles. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020. Springer International Publishing; 2020. pp. 251–259.
[15]. Wu Y, Hong Y, Ahmad S, Lin W, Yap P-T. White Matter Tract Atlases of the Baby Brain. cdn-akamai6connex.com.
[16]. Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103: 411–426.
[17]. Tournier J-D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202: 116137.
[18]. Mori S, Crain BJ, Chacko VP, Van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1999;45: 265–269.
[19]. Tournier JD, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proceedings of the international society for magnetic resonance in medicine. Ismrm; 2010.
Figures