1691
Assessment of microstructural changes following pediatric traumatic brain injury by advanced diffusion imaging1Division of Development and Growth, Department of Paediatrics and Gynaecology-Obstetrics, University of Geneva, Geneva, Switzerland, 2Center for Biomedical Imaging, Animal Imaging Technology section, Federal Institute of Technology of Lausanne, Lausanne, Switzerland, 3Department of Anesthesia and Intensive Care, Pitié-Salpêtrière Hospital, Paris, France, 4PROTECT, INSERM, Université Paris Diderot, Sorbonne Paris Cité, Paris, France, 5Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Traumatic brain injury (TBI) on the immature brain can have dramatic consequences on cerebral development. Understanding the underlying lesions of this abnormal development is of high interest. In this work, we used a mouse model of pediatric TBI at Postnatal day 7 (P7 - impact acceleration model) and assessed the long-term subsequent microstructural damages (at P45) using DTI and NODDI at 9.4T. Severe changes in white matter and cortical developments were observed. In conclusion, DTI derived parameters as well as NODDI estimates allow an accurate determination of the location and extent of the brain lesions following TBI.
Introduction
Traumatic brain injury (TBI) on the immature brain can have dramatic consequences on cerebral development. The incidence rate of TBI for children under 4 years of age is higher than for the rest of the population. Indeed, subsequent cognitive and behavioral deficits following TBI are more severe and persistent for immature than mature brains [1]. As such, understanding the underlying lesions of this abnormal development is of high interest. In this work, we used a mouse model of pediatric TBI (impact acceleration model) and assessed the long-term subsequent microstructural damages using diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) at 9.4T.Material and Methods
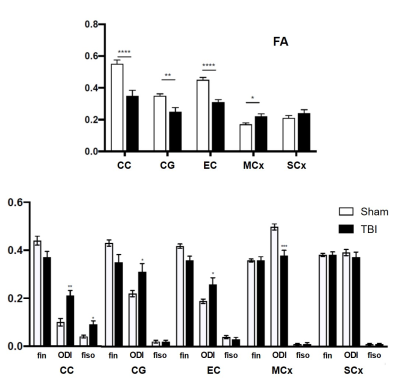
At Postnatal day 7 (P7, equivalent to human term) OF1 mice (Charles River, L’Arbresle, France) were anesthetized under isoflurane and subjected to a brain trauma (TBI group) by a closed head weight-drop trauma [2] with a skin incision. The skull was fixed into a stereotaxic frame and a foot-plate was placed on the center of the parietal bone (i.e. at the place of the skin incision, 2mm anterior and 1 mm lateral of the lambda). Contusion was induced by dropping a 10 g weight steel cylinder from a height of 10 cm onto the foot-plate. Sham group only had a skin incision under anesthesia. At P45 mice were sacrificed and brains were formalin-fixed for subsequent histology and ex-vivo MRI. MR experiments were performed on an actively-shielded 9.4T/31cm magnet (Agilent) equipped with 12-cm gradient coils (400mT/m, 120µs) with a 2.5 mm diameter birdcage coil. A multi-b-value shell protocol was acquired based on a spin-echo sequence with a matrix size of 64×64 on a FOV of 10×14 mm2. 12 slices of 0.6 mm thickness were acquired in the axial plane with 5 averages and TE/TR = 42/2000 ms. 96 diffusion weighted images were acquired, 15 of them as b0 reference images and 81 separated in 3 shells (δ/Δ=5.5/30 ms) with the following distribution (non-collinear and uniformly distributed in each shell): 21 directions with a b-value of 1750 s/mm2, 30 directions with a b-value of 3400 s/mm2 and 30 directions with a b-value of 5100 s/mm2. The total acquisition time was 15h per brain. Acquired data were fitted using DTI-TK for DTI and the NODDI toolbox [2] for NODDI. The diffusion tensor (DT) was spatially normalized to the study-specific DT template using DTI-TK [3]. The regions of interest (ROI) were manually delineated on direction encoded color maps and mean values of DTI derived parameters (Mean, Axial and Radial Diffusivities (MD, AD and RD) as well as Fractional Anisotropy (FA)) and NODDI estimates (intra-neurite volume fraction (fin), isotropic volume fraction (fiso) as well as Orientation Dispersion Index (ODI)) were calculated and averaged on the 6 following regions assessed: motor cortex (MCx) and somatosensory cortex (SCx) for grey matter; corpus callosum (CC), external capsule (EC) and cingulum (Cg) for white matter. For statistics (TBI vs. Sham), a Mann Whitney test was used (significance: *P<0.05).Results
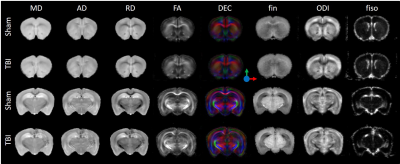
As depicted on figure 1, microstructural abnormalities were mainly observed close to the lesion site (at the level of hippocampus) and in the ipsilateral side. In all the white matter tracts assessed, a significant decrease of FA and increase of ODI was observed in the ipsilateral side of the TBI group compared to Sham (Figure 2). Indeed, fin tended to be lower in the WM of TBI compared to Sham. Only in CC, fiso increased in the TBI mice compared to Sham. In the other hand, in the grey matter FA significantly raised and ODI dropped in the MCx of TBI compared to Sham, whereas no difference was observed in the SCx.Discussion
MR diffusion probing of the mouse brain gives an accurate picture of the long-term microstructural changes following TBI. The induced contusion lead to focal lesions in the white and grey matter close to the impact site. TBI leads to WM abnormalities with disorganization of the fibers (FA and ODI) as well as reduction of axonal density (fin). Long-term necrosis and/or loss of matter was also observed (fiso). In the GM, MRI revealed a radial organization of the motor cortex that may be due to default in the dendritic arborization following TBI.Conclusion
In this study, we characterized the microstructural consequences of TBI in P7 pup mice recovered to P45. Severe changes in white matter and cortical developments were observed. Furthermore, DTI derived parameters as well as NODDI estimates allow an accurate determination of the location and extent of the brain lesions following TBI. These techniques may be of high interest for the clinical community of neonatologists due to their translational utility.Acknowledgements
Supported by the CIBM of the UNIGE, HUG, CHUV, EPFL, UNIL and Leenards and Jeantet foundations.References
[1] Role of microglia in a mouse model of paediatric traumatic brain injury, Chhor et al. Brain, Behavior, and Immunity, 2017.
[2] Subacute proteome changes following traumatic injury of the developing brain: Implications for a dysregulation of neuronal migration and neurite arborization. Kaindl et al. Proteomics Clin. Appl. 2007.
[3] NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Zhang H et al. Neuroimage 2012.
[4] Deformable registration of diffusion tensor MR images with explicit orientation optimization. Zhang H et al. Med. Image Anal. 2006.
Figures