1688
Diffusion Tensor Imaging in Cubital Tunnel Syndrome1University of Leeds, Leeds, United Kingdom, 2University of Manchester, Manchester, United Kingdom, 3Leeds Teaching Hospitals, Leeds, United Kingdom
Synopsis
Cubital Tunnel Syndrome (CuTS) is the 2nd most common compressive neuropathy affecting 6% of the population. Surgical decompression is the most effective treatment, but clinicians lack a reliable test to select patients for surgery. Diffusion tensor imaging (DTI) characterises tissue microstructure and so, DTI was acquired from 14 controls and 8 patients awaiting surgery in this proof-of-concept study. Patients had a significantly lower FA and higher RD than controls, throughout the length of the ulnar nerve. Therefore, DTI may add objective evidence of the ‘health’ of the ulnar nerve and aid the management of cubital tunnel syndrome.
Introduction
Cubital Tunnel Syndrome (CuTS) is the 2nd most common compressive neuropathy, affecting 36 per 100,000 person years[1] or 6% of the population[2]. Chronic compression leads to distortion of the axonal architecture, demyelination and fibrosis[3,4]. Surgical decompression is the most effective treatment and approximately 15,000 people per annum undergo surgical decompression in the UK[5] and USA[6]. However, clinicians lack a reliable test to select patients for surgery[7,8] and consequently, surgical decompression is unbeneficial in 13% of patients[9]. Furthermore, there is no non-invasive test which can objectively assess the ‘health’ of the ulnar nerve after surgery.[10] Diffusion tensor imaging (DTI) characterises tissue microstructure and provides reproducible[11–14] proxy measures of nerve ‘health’ which are sensitive to myelination, axon diameter, fibre density and organisation[15,16]. This study investigates the differences in the DTI metrics of the ulnar nerve between asymptomatic adults and those with CuTS (awaiting surgery).Methods
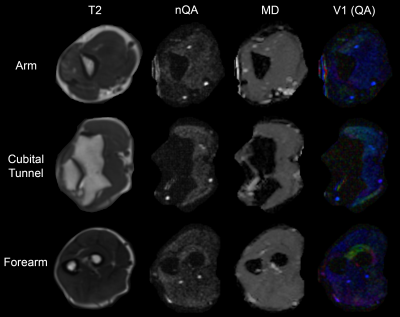
Asymptomatic adults and consecutive patients with a recent diagnosis of CuTS who were scheduled for decompressive surgery in our institution were recruited between July and November 2019. DTI was acquired at 3.0 tesla using single-shot echo-planar imaging (55 axial slices, 3 mm thick, 1.5 mm2 in-plane) with 30 diffusion sensitising gradient directions, a b-value of 800 s/mm2 and 4 signal averages. The sequence was repeated with the phase-encoding direction reversed. Data were combined and corrected in FSL[17] and imported to DSI Studio. Diffusion was quantified using restricted diffusion imaging[18] and reconstructed using generalised q-sampling imaging[19] (Figure 1). From every slice, the fractional anisotropy (FA), normalised quantitative anisotropy (nQA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) were extracted from 3 mm2 regions of interest covering the ulnar nerve. Metrics between patients with CuTS and controls were compared using mixed-effects linear regression.Results
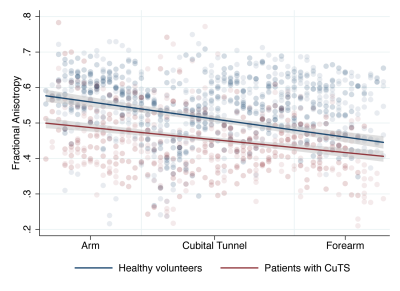
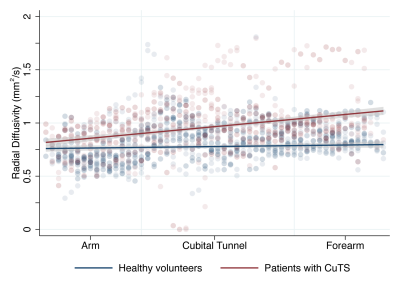
Thirteen controls (8 males, 5 females) and 8 patients with CuTS (6 males, 2 females) completed the study. Patients had a significantly lower FA than controls (mean difference 0.031 [95% CI 0.014, 0.048]; Figure 2) with the largest disparity in the arm (mean difference 0.087 [95% CI 0.035, 0.141]). Patients also had a significantly higher RD through the length of the ulnar nerve than controls (mean difference 0.252 [95% CI 0.085, 0.419]) with the largest disparity observed in the forearm (mean difference 0.252 x 10-4 mm2/s [95% CI 0.085 x 10-4, 0.419 x10-4]; Figure 3). There were no significant differences between patients and controls in QA, MD or AD. There was strong agreement between raters (ICC 0.02 [95% CI 0.002, 0.11]) as the variance in FA due to the person performing the analysis was 0.005 (95% CI 0.002, 0.01).Conclusions
This study shows that some diffusion tensor imaging metrics of the ulnar nerve in adults with CuTS are significantly different to those of asymptomatic adults. Moreover, these differences appear to manifest throughout the length of the ulnar nerve, not just at the supposed site of compression within the cubital tunnel. These findings suggest that DTI may add objective evidence of the ‘health’ of the ulnar nerve and aid in the management of cubital tunnel syndrome.Acknowledgements
No acknowledgement found.References
1. Hulkkonen S, Lampainen K, Auvinen J, Miettunen J, Karppinen J, Ryhänen J. Incidence and operations of median, ulnar and radial entrapment neuropathies in Finland: a nationwide register study. J Hand Surg (European Vol. 2019;175319341988674.
2. An TW, Evanoff BA, Boyer MI, Osei DA. The Prevalence of Cubital Tunnel Syndrome. J Bone Jt Surg. 2017;99:408–16.
3. NEARY D, EAMES RA. THE PATHOLOGY OF ULNAR NERVE COMPRESSION IN MAN. Neuropathol Appl Neurobiol. 1975;1:69–88.
4. Pham K, Gupta R. Understanding the mechanisms of entrapment neuropathies. Neurosurg Focus. 2009;26:E7.
5. NHS Digital. Hospital Admitted Patient Care Activity, 2017-18. 2018. Available from: https://digital.nhs.uk
6. Osei DA, Groves AP, Bommarito K, Ray WZ. Cubital Tunnel Syndrome: Incidence and Demographics in a National Administrative Database. Neurosurgery. 2017;80:417–20.
7. Greenwald D, Blum LC, Adams D, Mercantonio C, Moffit M, Cooper B. Effective surgical treatment of cubital tunnel syndrome based on provocative clinical testing without electrodiagnostics. Plast Reconstr Surg. 2006;117:87–91.
8. Kurver A, Smolders J, Verhagen WIM, Meulstee J, Nijhuis FAP. The Diagnostic Sensitivity for Ulnar Neuropathy at the Elbow Is Not Increased by Addition of Needle EMG of ADM and FDI When Nerve Conduction Studies Are Normal. Front Neurol. 2019;10:1–4.
9. Wade RG, Griffiths TT, Flather R, Burr NE, Teo M, Bourke G. Safety and Outcomes of Different Surgical Techniques for Cubital Tunnel Decompression. JAMA Netw Open. 2020;3:e2024352.
10. Chang K-V, Wu W-T, Han D-S, Özçakar L. Ulnar Nerve Cross-Sectional Area for the Diagnosis of Cubital Tunnel Syndrome: A Meta-Analysis of Ultrasonographic Measurements. Arch Phys Med Rehabil. 2018;99:743–57.
11. Nath V, Schilling KG, Parvathaneni P, Huo Y, Blaber JA, Hainline AE, et al. Tractography reproducibility challenge with empirical data (TraCED): The 2017 ISMRM diffusion study group challenge. J Magn Reson Imaging. 2020;51:234–49.
12. Vavasour IM, Meyers SM, Mädler B, Harris T, Fu E, Li DKB, et al. Multicenter Measurements of T 1 Relaxation and Diffusion Tensor Imaging: Intra and Intersite Reproducibility. J Neuroimaging. 2019;29:42–51.
13. Prohl AK, Scherrer B, Tomas-Fernandez X, Filip-Dhima R, Kapur K, Velasco-Annis C, et al. Reproducibility of Structural and Diffusion Tensor Imaging in the TACERN Multi-Center Study. Front Integr Neurosci. 2019;13:1–15.
14. Kimura M, Yabuuchi H, Matsumoto R, Kobayashi K, Yamashita Y, Nagatomo K, et al. The reproducibility of measurements using a standardization phantom for the evaluation of fractional anisotropy (FA) derived from diffusion tensor imaging (DTI). Magn Reson Mater Physics, Biol Med. Springer International Publishing; 2019;15–9.
15. Schmid AB, Campbell J, Hurley SA, Jbabdi S, Andersson JL, Jenkinson M, et al. Feasibility of Diffusion Tensor and Morphologic Imaging of Peripheral Nerves at Ultra-High Field Strength. Invest Radiol. 2018;53:705–13.
16. Friedrich P, Fraenz C, Schlüter C, Ocklenburg S, Mädler B, Güntürkün O, et al. The Relationship between Axon Density, Myelination, and Fractional Anisotropy in the Human Corpus Callosum. Cereb Cortex. 2020;30:2042–56.
17. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
18. Yeh F-C, Liu L, Hitchens TK, Wu YL. Mapping immune cell infiltration using restricted diffusion MRI. Magn Reson Med. 2017;77:603–12.
19. Fang-Cheng Yeh, Wedeen VJ, Tseng W-YI. Generalized q-Sampling Imaging. IEEE Trans Med Imaging. 2010;29:1626–35.
Figures