1685
Demyelination is related to deep gray matter iron deposition in CADASIL patients1radiology, Zhejiang University, hangzhou, China, 2radiology, the second affiliated hospital of zhejiang university, school of medicine, hangzhou, China
Synopsis
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a genetic cerebral small vessel disease. Apart from widespread white matter hyperintensities (WMHs), increased deep gray matter iron deposition was also suggested. Whether there is an association between white matter injury and deep gray matter iron deposition is undetermined. Iron deposition in deep gray matter was supposed due to the secondary degeneration followed by destroyed white matter integrity. Therefore, we investigate the iron deposition the relationship between white matter integrity and iron deposition to reveal the underlying mechanism of deep gray matter iron deposition in CADASIL patients.
Introduction
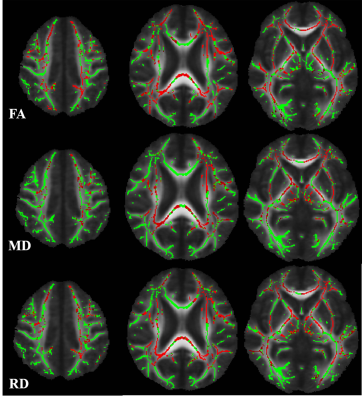
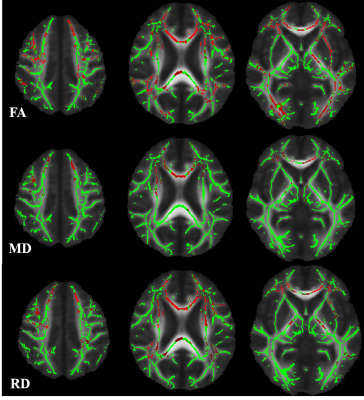
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a monogenic variant of cerebral small vessel disease caused by NOTCH3 mutation. It is characterized by widespread white matter hyperintensities (WMHs) on magnetic resonance imaging (MRI) of the brain. Apart from this, some studies indicated increased iron deposition in the deep gray matter in CADASIL patients. However, the mechanism underlying deep gray matter iron deposition in CADASIL patients has not been fully elucidated. Iron deposition in deep gray matter was supposed due to the secondary degeneration followed by destroyed white matter integrity. However, most of studies investigating the association between deep gray matter iron deposition and visible WMHs of presumed vascular origin found that there was no relationship between them. The visible WMHs of presumed vascular origin is consist of mixed pathological changes, including axonal damage, demyelination, and oedema. Therefore, the visible WMHs could not reflect underlying pathological changes and concealed the relationship between white matter injury and iron deposition. DTI is a powerful non-invasive technique to explore white matter integrity in total brain. Decreased fractional anisotropy(FA) and/or increased mean diffusivity(MD) measurements are commonly considered to be indicative of impaired white matter structural integrity—e.g., myelin damage, including demyelination; or disruption of tissue structure, including axonal damage. Furthermore, the directional diffusivity metrics axial diffusivity (AD), and radial diffusivity (RD), of white matter tracts have been hypothesized to differentiate axonal injury more specifically from demyelination in white matter tracts, respectively. Here, we investigated the iron deposition pattern in CADASIL patients based on 3-T MRI. Moreover, we used tract based spatial statistic (TBSS) method to identify the relationship between white matter integrity and deep gray matter iron deposition, therefore, to find out the potential mechanism behind the iron deposition in CADASIL patients.Methods
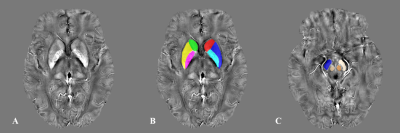
Forty-five patients with CADASIL and 73 healthy controls were included. Iron deposition in deep gray matter was evaluated using quantitative susceptibility mapping (QSM). We compared the iron deposition between groups and analyzed the correlations of visible WMHs and white matter microstructure with iron deposition in CADASIL patients.Results
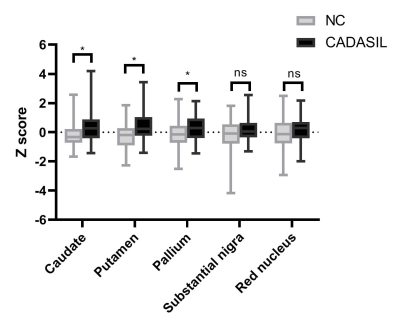
We found that compared with healthy control, CADASIL patients had increased iron deposition in putamen, caudate, and pallium. Besides, we found that iron deposition in putamen and pallium was related to decreased FA and increased MD, specifically the increased RD. We didn’t find the relationship between visible WMHs and deep gray matter iron deposition.Conclusion
In conclusion, we found that CADASIL patients showed increased iron deposition in deep gray matter based on 3-T MRI, and the demyelination is related to deep gray matter iron deposition in CADASIL patients.Acknowledgements
NoneReferences
Uchida Y, Kan H, Sakurai K, et al. Iron leakage owing to blood–brain barrier disruption in small vessel disease CADASIL[J]. Neurology, 2020, 95(9): e1188-e1198.
Sun C, Wu Y, Ling C, et al. Deep Gray Matter Iron Deposition and Its Relationship to Clinical Features in Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy Patients: A 7.0-T Magnetic Resonance Imaging Study[J]. Stroke, 2020, 51(6): 1750-1757.
Gattringer T, Khalil M, Langkammer C, et al. No evidence for increased brain iron deposition in patients with ischemic white matter disease[J]. Neurobiology of aging, 2016, 45: 61-63.
Liem M K, Oberstein S A J L, Versluis M J, et al. 7 T MRI reveals diffuse iron deposition in putamen and caudate nucleus in CADASIL[J]. Journal of Neurology, Neurosurgery & Psychiatry, 2012, 83(12): 1180-1185.
Valdes Hernandez M, Allerhand M, Glatz A, et al. Do white matter hyperintensities mediate the association between brain iron deposition and cognitive abilities in older people?[J]. European journal of neurology, 2016, 23(7): 1202-1209.
Figures