1679
Examining the association between sluggish cognitive tempo and functional connectivity in children with ADHD: A pilot study1Imaging Research Center, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Behavioral Medicine and Clinical Psychology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Sluggish cognitive tempo (SCT) is a behavioral phenotype characterized by excessive daydreaming that is frequently present in children with ADHD. This study examined functional connectivity in children with ADHD and high SCT, children with ADHD and low SCT, and typically developing children. Resting state fMRI data were acquired and analyzed to examine group differences as well as associations with ordinal ADHD and SCT symptom scores. Three a priori seeds were generated from NeuroSynth to investigate connectivity patterns associated with default mode, attention, and orienting domains. SCT scores were negatively associated with connectivity between attentional and medial visual areas.
Purpose
There is growing evidence that children’s attention problems are heterogeneous, with increasing interest in a sluggish cognitive tempo (SCT) phenotype characterized by excessive daydreaming, mental confusion, and slowed behavior/thinking. Approximately 40% of children with attention-deficit/hyperactivity disorder (ADHD) also experience symptoms of sluggish cognitive tempo (SCT)1. SCT refers to excessive daydreaming, mental confusion, and slowed behavior/thinking. Despite the prevalence of SCT and links to functional impairment, neural correlates remain almost entirely unexamined2. The present study examined functional connectivity in children with ADHD with and without co-occurring SCT, in addition to typically developing (TD) children with neither ADHD nor SCT.Material & Methods
Participants (TD=22, ADHD-SCT=23, ADHD+SCT=20, age=10.03 ± 1.48 yrs, 36 males, 29 females) had SCT and ADHD symptom ratings obtained from parents (N=65), teachers (N=60), and child self-report (N=59). Participants underwent both T1w structural (TR/TE 8.052 ms, 3.68 ms, flip angle 8°, FOV 256 x 256 mm², slice thickness 1 mm, voxel size 1 x 1 x 1 mm³) and resting-state functional EPI (TR/TE 2000 ms, 30 ms, flip angle 75°, FOV 224 x 224 mm², slice thickness 3 mm, voxel size 3 x 3 x 2.8 mm³, scan duration of 600 seconds) scan acquisitions with a Philips 3T Achieva (Best, The Netherlands). Participants were instructed to keep their eyes open and look at a fixed cross (+) during the functional scan acquisition. The acquired T1w structural and functional EPIs underwent quality assurance via MRIQC3,4. Structural T1w images underwent preprocessing and surface mesh reconstruction via fmriprep3,5 and FreeSurfer6. Functional images were preprocessed with FSL’s FEAT7,8 – which included motion correction9, brain extraction10, and high pass filtering (0.1 Hz) followed by aggressive ICA-based cleanup with FSL’s FIX11–14. Volumetric functional data was then mapped to the corresponding surface mesh and registered to the HCP S1200 group average surface template using connectome workbench, ciftify15,16, and multimodal surface matching17,18. The surface mapped timeseries were then smoothed with a smoothing kernel of 4mm FWHM in addition to being demeaned. Quality control of the preprocessed functional data, and the volume-to-surface mapped functional data were assessed via visual inspection. Three seed regions of interest were obtained from NeuroSynth19 using the terms: attention, orienting, and default mode. Seeds were mapped to the HCP S1200 group average surface template using connectome workbench. We assessed group differences as well as correlations between the SCT symptom scores and seed regions of interest via dual regression20,21 and permutation testing using FSL’s PALM22,23. All analyses controlled for sex and age at scan. Multiple regression analyses also included ADHD inattention scores in the models.Results
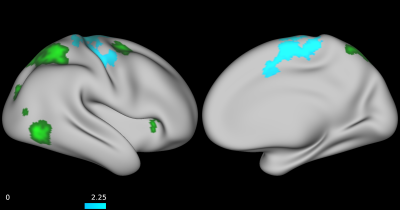
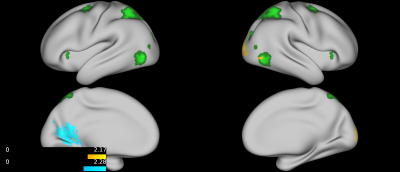
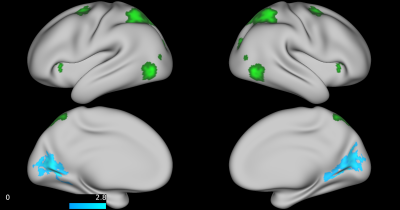
No group differences were found between TD, ADHD-SCT, and ADHD+SCT groups when controlling for age and sex. Parent-reported SCT symptoms were associated with decreased functional connectivity between the attention seed region and the portions of the brain centered around the pre- and post-central gyri in participants when controlling for age, sex, and parent-reported ADHD inattention symptoms (Figure 1). Teacher-reported SCT symptoms were associated with increased functional connectivity between the attention seed region and the right lateral portions of the visual pole, and decreased functional connectivity in the left lateral portion of the visual pole in participants when controlling for age, sex, and teacher-reported ADHD inattention symptoms (Figure 2). Child-reported SCT symptoms were associated with decreased functional connectivity between the attention seed region and two separate clusters. The first in the left hemisphere spanning portions of the: cuneus cortex, isthmus of the cingulate cortex, lingual gyrus, pericalcarine cortex, precuneus cortex. The second in the right hemisphere spanning portions of the: paracentral lobule, postcentral gyrus, precentral gyrus, superior frontal gyrus, superior parietal cortex (Figure 3).Discussion & Conclusion
This study, although preliminary, is one of the first to indicate that SCT symptoms may be associated with specific functional connectivity patterns independent of ADHD symptom severity. Specifically, both teacher- and child-rated SCT scores were negatively associated with connectivity between the attention seed region and visual processing regions. Additionally, parent-rated SCT scores were negatively associated with connectivity between the attention seed region and right lateral portions of the dorsal attention network. The default mode and orienting seed regions were not shown to have any significantly different functional connectivity patterns across the brain for reported SCT scores when controlling for age, and sex. Collectively, these findings suggest that SCT may be associated with disrupted processing between attentional and visual seed regions. Future directions should include further investigation into dorsal and ventral attention network mediated functional connectivity.Acknowledgements
This research was supported by a Cincinnati Children’s Research Foundation (CCRF) Trustee Award and the National Institute of Mental Health (NIMH; K23MH108603). The content is solely the responsibility of the authors and does not necessarily represent the official views of the CCRF or NIMH.References
1. Servera M, Sáez B, Burns GL, Becker SP. Clinical differentiation of sluggish cognitive tempo and attention-deficit/hyperactivity disorder in children. J Abnorm Psychol. 2018;127(8):818-829. doi:10.1037/abn0000375
2. Becker SP, Leopold DR, Burns GL, et al. The Internal, External, and Diagnostic Validity of Sluggish Cognitive Tempo: A Meta-Analysis and Critical Review. J Am Acad Child Adolesc Psychiatry. 2016;55(3):163-178. doi:10.1016/j.jaac.2015.12.006
3. Gorgolewski K, Burns CD, Madison C, et al. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front Neuroinform. 2011;5:13. doi:10.3389/fninf.2011.00013
4. Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12(9). doi:10.1371/journal.pone.0184661
5. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111-116. doi:10.1038/s41592-018-0235-4
6. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194. doi:10.1006/nimg.1998.0395
7. Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370-1386. doi:10.1006/nimg.2001.0931
8. Woolrich MW, Behrens TEJ, Smith SM. Constrained linear basis sets for HRF modelling using Variational Bayes. Neuroimage. 2004;21(4):1748-1761. doi:10.1016/j.neuroimage.2003.12.024
9. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825-841. doi:10.1016/S1053-8119(02)91132-8
10. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-155. doi:10.1002/hbm.10062
11. Beckmann CF, Smith SM. Probabilistic Independent Component Analysis for Functional Magnetic Resonance Imaging. IEEE Trans Med Imaging. 2004;23(2):137-152. doi:10.1109/TMI.2003.822821
12. Beckmann CF, Deluca M, Devlin JT, Smith SM. Investigations into Resting-State Connectivity Using Independent Component Analysis.
13. Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449-468. doi:10.1016/j.neuroimage.2013.11.046
14. Griffanti L, Salimi-Khorshidi G, Beckmann CF, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state seed region imaging. Neuroimage. 2014;95:232-247. doi:10.1016/j.neuroimage.2014.03.034
15. Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105-124. doi:10.1016/j.neuroimage.2013.04.127
16. Dickie EW, Anticevic A, Smith DE, et al. Ciftify: A framework for surface-based analysis of legacy MR acquisitions. Neuroimage. 2019;197:818-826. doi:10.1016/j.neuroimage.2019.04.078
17. Robinson EC, Jbabdi S, Glasser MF, et al. MSM: A new flexible framework for multimodal surface matching. Neuroimage. 2014;100:414-426. doi:10.1016/j.neuroimage.2014.05.069
18. Robinson EC, Garcia K, Glasser MF, et al. Multimodal surface matching with higher-order smoothness constraints. Neuroimage. 2018;167:453-465. doi:10.1016/j.neuroimage.2017.10.037
19. Tor D. W. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Front Neuroinform. 2011;5. doi:10.3389/conf.fninf.2011.08.00058
20. Nickerson LD, Smith SM, Öngür D, Beckmann CF. Using dual regression to investigate seed region shape and amplitude in functional connectivity analyses. Front Neurosci. 2017;11(MAR):115. doi:10.3389/fnins.2017.00115
21. Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/DualRegression?action=AttachFile&do=view&target=CB09.pdf. Published March 16, 2012. Accessed December 12, 2020.
22. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381-397. doi:10.1016/j.neuroimage.2014.01.060
23. Alberton BAV, Nichols TE, Gamba HR, Winkler AM. Multiple testing correction over contrasts for brain imaging. Neuroimage. 2020;216:116760. doi:10.1016/j.neuroimage.2020.116760
Figures