1675
Free-Water Eliminated DTI Measures of Neuro-inflammation and White Matter Structural Deterioration in HIV-1 Clade C Infection1University of Miami, Miami, FL, United States, 2Post Graduate Institute of Medical Education & Research, Chandigarh, India
Synopsis
Few neuroimaging studies have focused on HIV-1 clade C infection (HIV-1C) which comprises approximately half the global HIV population. In this work, we use a free-water eliminated diffusion tensor imaging (FWE-DTI) data processing approach to determine the extent of micro-structural brain damage in drug-naïve HIV-1C subjects. Our results show white matter (WM) structural abnormalities among HIV-1C subjects, manifested by increases in free water volume throughout the brain and reduced FWE fractional anisotropy (FWE-FA) along WM tracts. Our FWE-DTI approach provides an improved method for more accurate measures of brain abnormalities due to neuro-inflammation in HIV and other infections.
Introduction
Human immunodeficiency virus (HIV-1) enters the brain early in the course of infection, which triggers inflammation that gradually causes damage to the structural integrity of the brain, and results in HIV-1 associated mild-to-moderate neurocognitive disorders. However, the neurological outcomes in individuals infected by different clades of HIV-1 remain incompletely understood1,2,3,4. HIV-1 clade C (HIV-1C) comprises nearly half the global HIV population, and is the predominant strain found in South Africa, India, Brazil and China. Yet few neuroimaging studies have focused on HIV-1C compared to other clades such as clade B, which exists primarily in North America, Australia, and Western Europe where most of the research is performed. In this study, our aim is to evaluate the magnitude of micro-structural damage and neuro-inflammation in the brains of treatment naive HIV-1C subjects. We performed a whole-brain level data analysis using free-water eliminated DTI (FWE-DTI)5,6, a novel technique that can improve the accuracy of DTI metrics, and additionally provide the free-water volume fraction (fFW) as a quantification measure of the extra-cellular water contained in a voxel.Methods
Acquisition: Data were collected at the Post Graduate Institute of Medical Education & Research (PGIMER) in India from 45 treatment naive HIV-1C subjects (25/20 male/female; mean age: 31.33 ± 6.2; plasma CD4 count: 365.31 ± 202.4; Log10 plasma HIV viral load: 4.28 ± 0.8), and 45 age-range matched controls (27/18 male/female; mean age: 29.51 ± 7.2). We acquired whole-brain diffusion-weighted (DW) MRI on a 3T Siemens scanner, and collected 60 dual-shell (b = 1000/2000 s/mm2) DW-images using 30 gradient directions per shell for FWE-DTI analysis with the following parameters: 3×3×3 mm spatial resolution, TR = 1150 ms, TE = 98 ms, 54 axial slices.Processing: DW images are first pre-processed using FSL’s eddy correction and co-registration tools to correct for distortions. FWE-DTI fitting was performed using in house software based on the method defined by Hoy et al.6, where the FWE-DTI model is described as a bi-exponential expansion of DTI
$$S_{i}=S_{0}\left[\left(1-f_{FW}\right)exp\left(-b_{i}\cdot g_i^2D_{tissue}g_{i}\right)+f_{FW}\cdot exp\left(-b\cdot D_{iso}\right)\right]$$
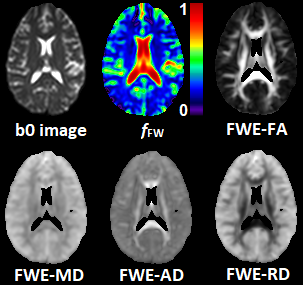
Here the contribution to the total signal Si is separated between two water compartments: free-water contained in the CSF and the extracellular space (with isotropic diffusion tensor Diso and volume fraction fFW), and tissue water contained in the intracellular space (with anisotropic diffusion tensor Dtissue and volume fraction 1- fFW). The FWE-DTI fitting problem aims at solving for Dtissue and fFW for each voxel as defined in equation above. From the tensor Dtissue we can find the FWE corrected metrics show in Figure 1, such as fractional anisotropy (FWE-FA), mean diffusivity (FWE-MD), axial diffusivity (FWE-AD), and radial diffusivity (FWE-RD).
Analysis: We analyzed the processed data at the whole brain level using the JHU-MNI-SS type2 atlas7 from which we selected 26 white matter (WM) and 20 grey matter (GM) regions of interest (ROI). We evaluated fFW and FWE-MD for the whole-brain, while FWE-FA, FWE-AD, and FWE-RD were evaluated at WM ROIs only. We compared these measures between control and HIV-1C subjects using a t-test to assess between-group differences. Finally, we ran a Pearson correlation test for the HIV-1C group to find associations between the FWE-DTI metrics and laboratory indices such as CD4 cell count and plasma HIV viral load (VL). The significance threshold was set at p < 0.05, corrected for multiple comparisons using false discovery rate (FDR).
Results
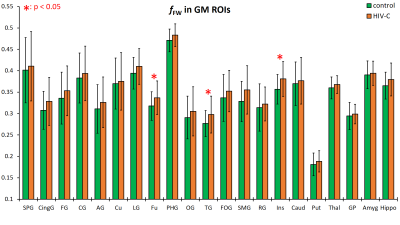
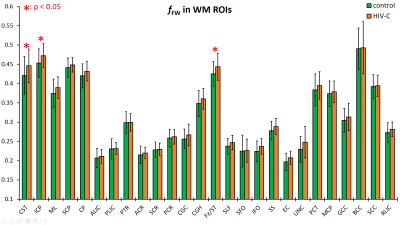
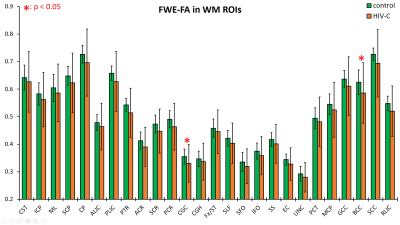
Data from the HIV-1C subjects showed consistently increased fFW in both the GM and WM ROIs spread throughout the brain. In GM ROIs (Figure 2), the fFW increases were most significant in the fusiform gyrus (Fu), temporal gyrus (TG), and insular gyrus (Ins), while in WM ROIs (Figure 3) the fFW increases was observed in the corticospinal tract (CST), inferior cerebellar peduncle (ICP), and the Fornix (Fx). We also found FWE-FA consistently decreasing for the HIV-1C subjects across all WM tracts (Figure 4), with significant decreases in the cingulum (CGC) and in the body of the corpus callosum (BCC). Other DTI metrics did not show significant differences (p > 0.05) or noticeable trends between the two groups. Also, we did not find any significant correlation between CD4 count or VL, and the measured DW-metrics for any ROI.Discussion
The reduction in FWE-FA in WM regions is evidence of damage to WM integrity in the brains of HIV-1C subjects. Additionally, elevated fFW is indicative of an expansion of the extra-axonal space and is generally accepted as a sign of neuro-inflammation. However, some studies have also shown reduced volumetric measures in multiple GM and WM structures in the brains of HIV-1C subjects8. Therefore, we speculate that the increase in free water fraction is not contributing to an increase in total brain volume, but rather this increased volume is compensated by concomitant reduction in axonal density or WM atrophy. Our findings show that HIV-1C infection can cause extensive damage to the brain despite reports of lower neuro-virulence compared to other HIV clades. The present study is part of a larger research effort using multimodal MRI techniques as well as neurocognitive testing to understand the effects of HIV-1C infection to the brain.Acknowledgements
Funding from NIH grant, R01 NS094043.References
1. Tyor W, et al. Effect of HIV clade differences on the onset and severity of HIV-associated neurocognitive disorders. J Neurovirol. 2013 Dec;19(6):515-22.
2. Paul RH, et al. Neuroimaging abnormalities in clade C HIV are independent of Tat genetic diversity. J Neurovirol. 2017 Apr;23(2):319-328.
3. Constantino AA, et al. HIV-1 clade B and C isolates exhibit differential replication: relevance to macrophage-mediated neurotoxicity. JC Neurotox Res. 2011 Oct; 20(3):277-88.
4. Hoare J, et al. White-Matter damage in Clade C HIV-positive subjects: a diffusion tensor imaging study. J Neuropsychiatry Clin Neurosci. 2011 Summer;23(3):308-15.
5. Pasternak O, et al. Estimation of extracellular volume from regularized multi-Shell diffusion MRI. Med Image. 2012;15:305-312.
6. Hoy AR, et al. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. NeuroImage 2014;103:323-333.
7. Oishi K, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage;2009;46(2):486-99.
8. Ortega M, et al. HIV clades B and C are associated with reduced brain volumetrics. J Neurovirol. 2013 Oct;19(5):479-87.
Figures