1673
Assessment of brain structural connectome alterations in depressive patients with suicidal attempt using GQI1Department of Medical Imaging and Radiological Sciences, and Bachelor Program in Artificial Intelligence, Chang Gung University, Taoyuan, Taiwan, 2Department of Psychiatry, Chang Gung Memorial Hospital, Chiayi, Taiwan, 3School of Medicine, Chang Gung University, Taoyuan, Taiwan, 4Department of Diagnostic Radiology, Chang Gung Memorial Hospital, Chiayi, Taiwan, 5Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
Synopsis
Depression is a key factor in committing suicide. Patients with depression often accompany with brain network alterations. Three groups of participants were recruited in the study, including healthy controls (HC), depressed patients with and without suicidal attempt history (SA, D). We analyzed brain network alternations by using their GQI data. SA group showed lower global integration and higher local segregation compared to D and HC groups. Furthermore, SA and D groups had significant subnetwork connections in frontal and parietal lobes than HC group. The alternations were found in the network measurement in depressed patients with and without suicidal attempt history.
Introduction
Depression is one of the causes of disability with highly prevalent and recurrent 1,2. People with depression encounter deficiency in emotion processing, memory, and executive function 3, and carry an increased risk of suicide 4. Studies showed that the most important risk factor of completing suicide is suicidal attempt 5. Previous studies mainly focused on biologically differences and physically behaviors 6,7. However, brain network alterations are important factors for patients with depression and suicidal attempts 8. We adopted a unique diffusion MRI reconstruction approach called generalized q-sampling imaging (GQI), which can improve the accuracy of fiber orientation, and shows good specificity and sensitivity for the evaluation of white matter integrity 9. We also used graph theoretical approach to explore complicated small-world brain network changes, including integrated and segregated functional characteristics 10.Methods
155 participants were recruited from Chiayi Chang Gung Memorial Hospital and assigned into three groups: 44 depressed patients with suicidal attempt history (SA), 56 depressed patients without suicidal attempt history (D), and 55 healthy controls (HC). Inclusion criteria were aged over 20 years old and right-handed. Exclusion criteria were MRI contraindications, pregnancy, history of severe brain damage and other mental disorders. Participants were scanned by 3T MRI system (Verio, SIEMENS, Germany). Image acquisition parameters were repetition time/echo time = 8943/115 msec, number of excitations = 1, field of view = 250 × 250 mm2, slice thickness = 4mm, matrix size = 128 × 128, voxel size = 3.4 × 3.4 × 4 mm3, b-values = 0, 1000, 1500 and 2000 s/mm2 in 193 non-collinear directions.In the graph theoretical analysis (GTA), we reconstructed pathways of brain neural fibers using DSI Studio and calculated topological properties including global integration, local segregation and small-worldness index of brain networks. The area under curve (AUC) of topological parameters was compared among groups. The network-based statistical (NBS) analysis was used to identify differences of connected brain subnetworks between groups. The BrainNet viewer was then used to identify and picture significant sub-networks.
Results
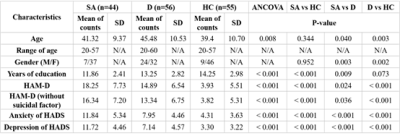
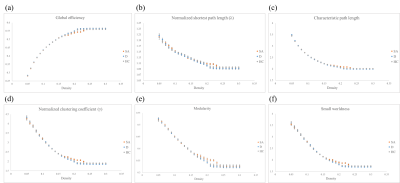
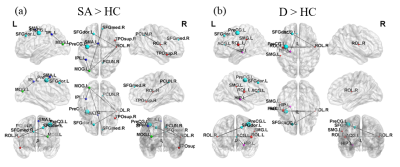
Figure 1 showed the demographic characteristic of participants. Age, gender, years of education, the score of Hamilton Rating Scale for Depression (HAM-D) without suicidal factor and the score of Hospital Anxiety and Depression Scale (HADS) were considered as covariates to adjust the impact of each factor for analysis to investigate differences among groups.Both D and HC groups’ networks showed similar tendencies in global efficiency, normalized shortest path length (λ), characteristic path length, normalized clustering coefficient (γ), modularity (Fig. 2a to 2f). However, SA group illustrated significantly lower global efficiency as well as longer λ and characteristic path length (Fig. 2a to 2c). It also demonstrated significantly higher γ and modularity (Fig. 2d and 2e). Our results showed that small-worldness index of all three groups were greater than 1. SA group had stronger subnetwork connections in both frontal and parietal lobes than HC group (Fig. 3a). Moreover, D group demonstrated stronger subnetwork connections in the parietal lobe than HC group (Fig. 3b).
Discussion
GTA results showed that D and HC groups had similar network tendencies without significant differences among 6 topological parameters, while SA group had significant differences compared to D and HC groups. First, our analyses showed that small-world property remained in three groups (small-worldness index > 1). SA group had a lower global efficiency that represents the reduction of global integration. Our study also demonstrated increasing γ and modularity of SA group, which represented that SA group had better local segregation.Regular networks are networks with nodes connected to the nearest neighbors, which represents high local segregation and turns out the same as SA group in our study. It may associate with lower verbal memory scores 11. However, brain is proposed to work economically 12, which makes regular networks less efficient. SA and D groups showed few connections between brain regions that are more notable than HC group, especially the frontal and parietal lobes. Previous studies have demonstrated that frontothalamic loops were abnormal in depressed patients with suicide history 13.
Conclusion
Our results showed that network alternations are related to patients with depression and suicidal attempt. Our study analyzed network differences in healthy controls, depressed patients with and without suicidal attempt history and found variously significant results, which adequately supported to previous studies. More researches can be conducted in the future to have a further understanding.Acknowledgements
This study was supported by the research programs, MOST106-2314-B-182-040-MY3 and MOST109-2314-B-182-047-MY3, which were sponsored by the Ministry of Science and Technology, Taipei, Taiwan.References
1. Murray, C. J.; Vos, T.; Lozano, R. et. al., Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380 (9859), 2197-223.
2. Richards, D., Prevalence and clinical course of depression: a review. Clinical psychology review 2011, 31 (7), 1117-25.
3. McIntyre, R. S.; Cha, D. S.; Soczynska, J. K.; Woldeyohannes, H. O.; Gallaugher, L. A.; Kudlow, P.; Alsuwaidan, M.; Baskaran, A., Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depression and anxiety 2013, 30 (6), 515-27.
4. Li, H.; Luo, X.; Ke, X.; Dai, Q.; Zheng, W.; Zhang, C.; Cassidy, R. M.; Soares, J. C.; Zhang, X.; Ning, Y., Major depressive disorder and suicide risk among adult outpatients at several general hospitals in a Chinese Han population. PloS one 2017, 12 (10), e0186143.
5. Bostwick, J. M.; Pabbati, C.; Geske, J. R.; McKean, A. J., Suicide Attempt as a Risk Factor for Completed Suicide: Even More Lethal Than We Knew. The American journal of psychiatry 2016, 173 (11), 1094-1100.
6. Sotelo, J. L.; Musselman, D.; Nemeroff, C., The biology of depression in cancer and the relationship between depression and cancer progression. International review of psychiatry 2014, 26 (1), 16-30.
7. Cuijpers, P.; Quero, S.; Dowrick, C.; Arroll, B., Psychological Treatment of Depression in Primary Care: Recent Developments. Current psychiatry reports 2019, 21 (12), 129.
8. Bani-Fatemi, A.; Tasmim, S.; Graff-Guerrero, A.; Gerretsen, P.; Strauss, J.; Kolla, N.; Spalletta, G.; De Luca, V., Structural and functional alterations of the suicidal brain: An updated review of neuroimaging studies. Psychiatry research. Neuroimaging 2018, 278, 77-91.
9. Yeh, F. C.; Wedeen, V. J.; Tseng, W. Y., Generalized q-sampling imaging. IEEE transactions on medical imaging 2010,29 (9), 1626-35.
10. Shine, J. M., Neuromodulatory Influences on Integration and Segregation in the Brain. Trends in cognitive sciences 2019, 23 (7), 572-583.
11. Hwang, J.; Legarreta, M.; Bueler, C. E.; DiMuzio, J.; McGlade, E.; Lyoo, I. K.; Yurgelun-Todd, D., Increased efficiency of brain connectivity networks in veterans with suicide attempts. NeuroImage. Clinical 2018, 20, 318-326.
12. Dusi, N.; Barlati, S.; Vita, A.; Brambilla, P., Brain Structural Effects of Antidepressant Treatment in Major Depression. Current neuropharmacology 2015, 13 (4), 458-65.
13. Bullmore, E.; Sporns, O., The economy of brain network organization. Nature reviews. Neuroscience 2012, 13 (5), 336-49.
Figures