1666
MRI measurements demonstrate gray matter increases induced by transcranial direct current stimulation treatment in depression1UCLA, Los Angeles, CA, United States, 2University of Alabama, Birmingham, Birmingham, AL, United States
Synopsis
Transcranial direct current stimulation (tDCS) is a low-cost and non-invasive neuromodulation technique. Although tDCS has been shown to improve symptoms in psychiatric disorders, the neurobiological effects of tDCS are not well-understood. In this study, we used MRI to investigate structural changes in the brain resulting from tDCS. T1-weighted MRI data from n=59 depressed participants was acquired pre/post tDCS treatment, and analysis revealed gray-matter increases near the stimulation-target and in a distant but functionally-connected region. These results indicate that tDCS treatments can elicit structural changes in the brain; both near the stimulation-target and in additional regions part of the same brain-network.
INTRODUCTION:
Transcranial direct current stimulation (tDCS) is a low-cost and non-invasive neuromodulation technique that uses milliampere electric currents applied at the scalp to modulate cortical excitability 1. Although tDCS has been shown to improve symptoms in neurologic and psychiatric disorders 2,3, recent contrasting findings from clinical trials 4,5, and meta-analyses 6 have highlighted the need to understand the underlying neurobiological effects of tDCS. In this study, we used MRI to investigate whether a tDCS treatment series induces gray matter structural plasticity in depressed participants.METHODS:
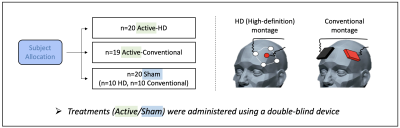
Using a double-blind parallel design, a total of 59 moderately depressed participants were randomized to receive active/sham tDCS treatments via conventional or high-definition (HD) tDCS montages (Fig. 1, n=20/19/20 in active-HD, active-conventional, and sham groups, respectively). Both tDCS montages were individualized using Brainsight neuronavigation 7 to deliver tDCS currents at the left dorsolateral prefrontal cortex (DLPFC). The HD-montage utilized a common 4x1 ring arrangement with the anode positioned over the stimulation-target and the cathodes placed 5cm away to deliver focal tDCS. In contrast, the conventional montage utilized commonly-used 7x5cm electrodes, with the anode positioned over the stimulation-target and the cathode over F8 (10-20 EEG coordinates). tDCS treatment consisted of twelve 2mA X 20min of active/sham stimulation sessions spread out over twelve consecutive working days. Stimulation was delivered using a double-blind stimulator (Soterix, Model#5100D) with the participants at rest. Sham-tDCS involved a ramp up to 2mA followed immediately by a ramp-down at the beginning of each treatment-session. Apart from these brief periods of ramping, ammeter readings showed that the device emitted a steady current of 65uA during sham-tDCS; note that this current is an order of magnitude smaller than active-stimulation. All ramp-times were 30 seconds. All subjects gave written informed consent, and study procedures were approved by the UCLA Institutional Review Board (IRB).MRI data was acquired pre and post-tDCS treatment for each subject using the Human Connectome Project (HCP 8) T1-weighted MPRAGE sequence (Sequence parameters: TE1/TE2/TE3/TE4 = 1.81/3.6/5.39/7.18 ms, TI=1s, TR=2.5s, 80 FA, 0.8x0.8x0.8 mm3 voxel, 320x320 matrix, 208 slices, 740 Hz/Px bandwidth for all TE’s, 6/8 partial Fourier, R=2 GRAPPA acceleration). All data was acquired on a Siemens 3T PRISMA MRI scanner using a 64-channel head coil.
Acquired T1-weighted MRI data was segmented using the computational anatomy toolbox (CAT12) and MATLAB. Next, gray matter difference maps (post minus pre treatment) were generated for each subject using voxel-based morphometry 9. Finally, these difference maps were analyzed using a voxelwise 1-way ANOVA to compare gray-matter changes between active-HD, active-conventional and sham groups. Note that sham-HD and sham-conventional participants were combined to form a single “sham” group since no significant differences were observed between these groups.
RESULTS:
Results revealed significant (p < 0.05 FDRc corrected) gray-matter changes at the left-DLPFC stimulation-target and in the posterior cingulate cortex (PCC), with post-hoc t-tests revealing significant increases for both active-stimulation groups compared to sham at the left DLPFC, and significant increases at the PCC only for the active-HD group (Fig 2, Left DLPFC change: active-HD: p=0.0002, cohen’s d = 1.3, active-conventional: p = 0.045, d = 0.59. PCC change: active-HD: p = 0.01, d = 0.97; active-conventional p = 0.99, d = -0.01). While no significant differences between the active-stimulation montages were observed at the left DLPFC stimulation-target, significant differences between the active-HD and active-conventional groups were observed at the PCC (active-HD vs. active-conventional : Left DLPFC: p = 0.10, d = 0.71; PCC: p = 0.01, d = 0.92).DISCUSSION AND CONCLUSION:
TDCS treatment resulted in gray-matter increases at the left DLPFC stimulation-target, similar to prior reports of structural neuroplasticity occurring with other neurostimulation treatments for depression, including electroconvulsive therapy and transcranial magnetic stimulation 10,11. Additionally, significant increases in gray matter were also observed with the active-HD montage at the PCC. Because the HD-montage induces currents that are more spatially focused compared to the conventional montage, the finding of stronger gray-matter increases at distal brain regions indicates the spread of neuromodulatory effects through functional brain networks independent of the applied currents 12. Indeed, the PCC is an association region well-known to be functionally-connected with the left DLPFC 13. Overall, these results demonstrate that tDCS treatments can elicit structural changes in the brain; both near the stimulation-target and in additional brain-regions that form a functional brain-network. Future work will investigate treatment-induced mood-improvements and their correlations with the observed gray-matter increases.Acknowledgements
The work was supported by NIH grant R61MH110526
References
1. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3:633-9.
2. Kuo MF, Chen PS, Nitsche MA. The application of tDCS for the treatment of psychiatric diseases. International review of psychiatry. 2017;29(2):146-67.
3. Jog MV, Wang DJJ, Narr KL. A review of transcranial direct current stimulation (tDCS) for the individualized treatment of depressive symptoms. Pers Med Psychiatry. 2019;17-18:17-22.
4. Loo CK, Husain MM, McDonald WM, Aaronson S, O'Reardon JP, Alonzo A, et al. International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimul. 2018;11(1):125-33.
5. Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N Engl J Med. 2017;376(26):2523-33.
6. Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul. 2015;8(3):535-50.
7. Brainbox-Neuro. Brainsight Neuronavigation [Available from: https://brainbox-neuro.com/catalogue/neuro-navigation/tms-navigation/brainsight-tms-navigation/.
8. Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. NeuroImage. 2018;183:972-84.
9. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839-51.
10. Mulders PCR, Llera A, Beckmann CF, Vandenbulcke M, Stek M, Sienaert P, et al. Structural changes induced by electroconvulsive therapy are associated with clinical outcome. Brain Stimul. 2020;13(3):696-704.
11. Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial Magnetic Stimulation of Left Dorsolateral Prefrontal Cortex Induces Brain Morphological Changes in Regions Associated with a Treatment Resistant Major Depressive Episode: An Exploratory Analysis. Brain Stimul. 2016;9(4):577-83.
12. Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(41):E4367-75.
13. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253-8.
Figures