1663
Circadian rhythm functional network in the central nervous system and its disruptions in chronic insomnia disorder
Ran Pang1,2, Jianli Wang3, Karunanayaka Prasanna3, Kuncheng Li4, and Qingxian Yang2
1Department of Radiology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, 2Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, PA, United States, 3Department of Radiology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 4Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China
1Department of Radiology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, 2Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, PA, United States, 3Department of Radiology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 4Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China
Synopsis
The etiology of chronic insomnia disease (CID) ultimately relates to the asynchrony of circadian rhythms. Using the anterior hypothalamus as a seed, functional connectivity (FC) of the circadian rhythm functional network (CRFN) during resting state was demonstrated in healthy subjects, consisting of both positive and negative FCs in the cerebrum and cerebellum. The CID patients exhibited an extensive weakening of FCs and abnormal local hyperactivities, reflecting underlying asynchrony in the CRFN.
INTRODUCTION
Chronic insomnia affects the life quality of 10-30% of the general population. Its etiology is complicated, but thought to ultimately relate to the asynchrony of circadian rhythms. The circadian rhythm functional network (CRFN) governs our daily life cycle, but remains poorly understood. Resting state fMRI, based on synchronization of spontaneous neuronal activity, is particularly suited for elucidating the synchrony of the CRFN in the human central nervous system (CNS). The suprachiasmatic nucleus (SCN), in the hypothalamus, is considered the pacemaker of CRFN that regulates wake-sleep cycle. The goals of this study is to determine the functional connectivity (FC) of the circadian rhythm function network during resting state in healthy controls (HCs) and evaluate its FC changes in patients with chronic insomnia disorder (CID).METHODS
Twenty-six CID and 18 HC subjects were recruited and studied. Their demographic and clinical information is provided in Table 1. Sleep quality was evaluated with Polysomnography (PSG) in 2 continuous nights, and subjective clinical measures of the Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index Scale (ISI) (Table 1). Resting-state fMRI (rs-fMRI) was conducted at 3T with an echo planar imaging (EPI) sequence. The FC of the CRFN was estimated using Data Processing Assistant for Resting-State fMRI (http://rfmri.org/DPARSF) with bilateral SCNs as seeds (Fig. 1A). 1 Resting-state parameters, fractional amplitude of low frequency fluctuations (fALFF)2 and regional homogeneity (ReHo)3 were estimated to characterize the local resting-state functional changes in CID. Correlation between resting-state functional parameters and clinical variables, such as, Mini-Mental State Examination, Pittsburgh Sleep Quality Index, Hamilton Anxiety Scale scores and PSG data was analyzed with SPSS.RESULTS
Functional connectivity analysis showed that the CRFN in HC consists of brain structures with positive and negative FCs with the SCN (Fig. 1, Table 1). The positive FC is clustered in the inferior-medial and -temporal lobes while the negative FC is more widespread into the frontal cortex, visual cortex (V1 and V3(BA19)), sensory-motor cortex and the cerebellum. Comparing to that in the HCs, the FC of CRFN was significantly weaker in the negative FC, especially, in the cerebellum (Table 1 and Fig. 2), which is accompanied with an augmented fALFF and ReHo (Fig. 3). Furthermore, the ReHo value of these regions of FC in CID patients, such as, bilateral cerebellum (8), left cerebellum (2) and left somatosensory cortex exhibited positive correlation with the insomnia clinical measures (PSQI and ISI) and neuropsychological parameters (HAMD) (p < 0.05, r > 0.5).DISCUSSIONS
The hypothalamus controls the circadian rhythm through a reciprocal feedback system via excitatory-inhibitory mechanisms in the circadian network, such as the retinohypothalamic tract, geniculohypothalamic tract and projections from Raphe nuclei. Consistent with this model, 4 the CRFN in this study includes the frontal, sensory-motor, visual cortex, LGN, raphe nuclei and structures in the cerebellum. Our finding of positive and negative FC is likely related to intricate excitatory-inhibitory networks, providing an adaptable yet stable basis for circadian rhythms. As demonstrated in the CID group, weakening or disruption of the synchrony of FCs among the subordinate brain structures presented abnormally augmented activities, which correlated with CID clinical symptoms, specifically, in the cerebellum.CONCLUSIONS
Our analysis revealed that the CRFN, consisting of positive and negative FCs in the cerebrum and cerebellum, could relate to excitatory-inhibitory regulatory networks. The CRFN in CID patients exhibited extensive changes that are correlated to their clinical expressions. Thus, delineating CRFN is important not only for understanding the circadian network in humans but also for developing objective markers for CID and other related sleeping disorders.Acknowledgements
We thank all authors of the included studies. We especially thank Center for NMR Research of Penn State University College of Medicine for all staffs' kind help and suggestion.References
- Boes, A. D., Fischer, D., Geerling, J. C., et al. Connectivity of sleep- and wake-promoting regions of the human hypothalamus observed during resting wakefulness. Sleep, 2018;41(9):1-12. 2.
- Zou, Q. H., Zhu, C. Z., Yang, Y., et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods, 2008;172(1):137-141.
- Jiang, L., Zuo, X. N. Regional Homogeneity: A Multimodal, Multiscale Neuroimaging Marker of the Human Connectome. The Neuroscientist, 2016;22(5), 486-505.
- Hastings, M. H., Maywood, E. S., Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nature reviews. Neuroscience, 2018;19(8): 453-469.
Figures
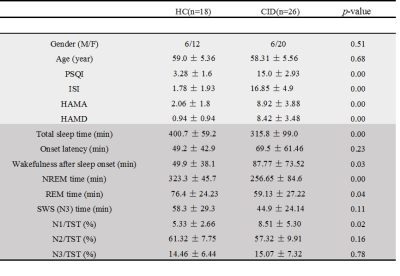
Table 1. Demographic, clinical, neuropsychological and PSG data of HC and CID
subjects.
Note: Chi-squared test for gender comparison; 2-sample t-test for the
other indexes’ comparisons between CID and HC groups. NREM: non-rapid eye movement; REM: rapid eye
movement; SWS (N3): slow wave activity; N1/TST: N1 sleep time/total sleep time
ratio; N2/TST: N2 sleep time/total sleep time ratio; N3/TST: N3 sleep
time/total sleep time ratio.
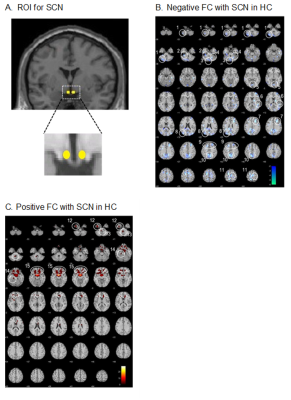
Fig. 1. A. ROI for SCN in bilateral
anterior hypothalamus. B. Negative FC with SCN in the HC subjects.
C. Positive FC with SCN in the HCs (p < 0.005, extent threshold = 5
voxels). The brain structures labeled 1 to 15 are listed in Table 2.
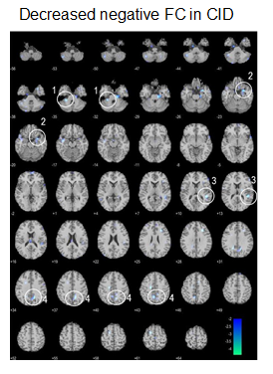
Fig. 2. Compared to HCs, CID subjects showed significantly
lower negative FC with the SCN (p < 0.005, extent threshold = 5 voxels).
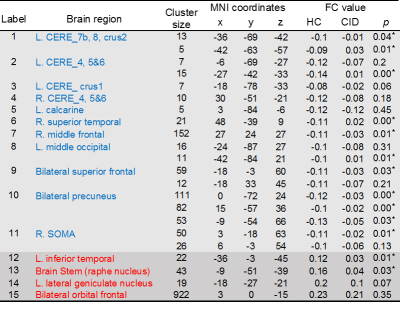
Table 2. Regions with negative (blue) and positive (red)
functional connectivity with the SCN in HCs (voxel ≥ 5, p < 0.005). Note:
2-sample t-test were used for the FCs’ comparisons between HC group and CID
group, *significant
difference between two groups (p<0.05).
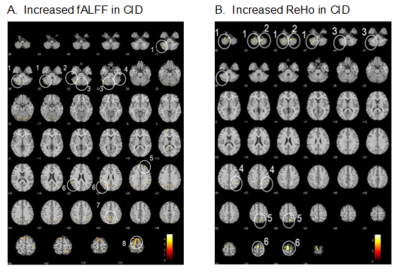
Fig. 3. Compared to HC, CID patients
showedsignificantly higher fALFF value (A, p < 0.005, extent
threshold = 5 voxels) and ReHo value (B, p < 0.001 extent threshold =
5 voxels).