1662
Disrupted Small-World Networks in Major Depressed Patients with Suicidality1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2Department of Psychiatry, West China Hospital of Sichuan University, Chengdu, China, 3Department of Nuclear Medicine, West China Hospital of Sichuan University, Chengdu, China
Synopsis
In this study, we investigated depressed suicidal brain from the level of network connection. We constructed brain structural networks using diffusion tensor imaging. Then all graph theoretical network parameters including small-world parameters (Cp, Lp, γ, λ and σ), network efficiency parameters (Eloc and Eglob) and nodal efficiency were analyzed. We found decreased Eloc/Eglob/Cp, increased Lp/λ and decreased nodal efficiency in fronto-striatal-limbic-thalamic circuit in depressed suicidal patients. In summary, suicidality involves complex neocortical network organization, which showed a weaker integration and disrupted fronto-striatal-limbic-thalamic circuit.
Background
Suicide takes a tremendous toll on global public health with an annual global age-standardized suicide rate of 11.4 per 100000 people [1]. Psychiatric disorders particularly depressed disorders constitute proximal risk factors of suicide. Diffusion tensor imaging (DTI) is an MRI-based neuroimaging method that can infer properties of structural brain connectivity in vivo [2]. The complex connectivity of structural networks could support higher cognitive and affective processes. Now we explore the structural alteration of brain in patients with suicidality and depression from the level of network connection.Methods
This study was approved by the Ethics Committee of the West China Hospital, Sichuan University, and all subjects provided written informed consent. Participants were included 51 healthy controls (HC), and 103 depressed patients including 47 patients without suicidality (patient controls, PC) and 56 patients with suicidality patients (SU). Clinical symptoms were assessed with the 17-item Hamilton Depression Rating Scale (HAMD-17) and Hamilton Anxiety Rating Scale (HAMA).Data analysis was performed as followed steps. 1) Preprocessing: Structural images were preprocessed using PANDA, a pipeline toolbox for analyzing brain diffusion images using the FSL (FMRIB Software Library toolbox) [3] implemented in MATLAB. The main procedures including brain extraction (BET), eddy-current correction and creation of the fractional anisotropy (FA) maps. 2) Network construction: We used the deterministic tractography extracted in PANDA to construct brain structural connectome. We defined a structural connectome for each participant comprised of a collection of nodes and edges interconnecting the nodes, where the nodes represented brain regions of the automated anatomic labeling atlas (AAL90), and the edges represented FA values of the fiber. In detail, the deterministic fiber tracking was defined to terminate when either FA was < 0.2 or turn angle > 45°. Thus, an average FA-weighted symmetrical anatomical 90 × 90 network matrix was obtained for each subject. 3) Network Analyses: all graph theoretical network analysis was performed in GRETNA. The threshold range was 0.10 < S < 0.40 with an interval of 0.01 to ensure the small-world index was larger than 1. Then area under the curve (AUC) was used to measure across the sparsity parameter S for each network metric. Global network measures included small-world parameters[4] (Cp, Lp, γ, λ and σ), network efficiency parameters [5] (Eloc and Eglob) and nodal efficiency. 4) Statistical analysis: analysis of variance (ANOVA) was used to compare AUC values of each metric among the three groups, followed by post-hoc tests. Statistical significance was set as p < 0.05. We performed a Pearson correlation analyses between these metrics and the HAMD, HAMA scores.
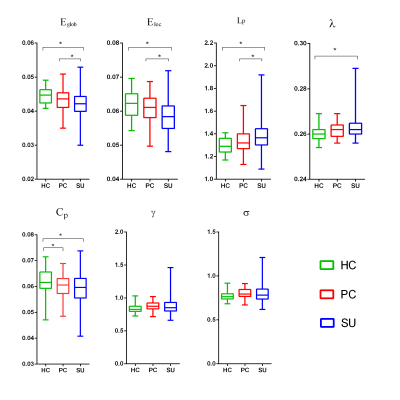
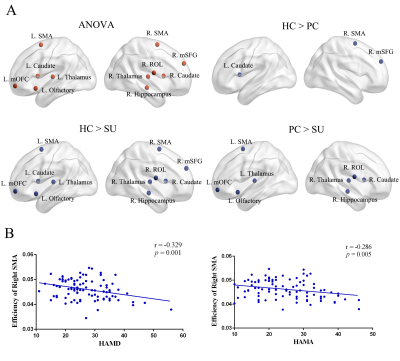
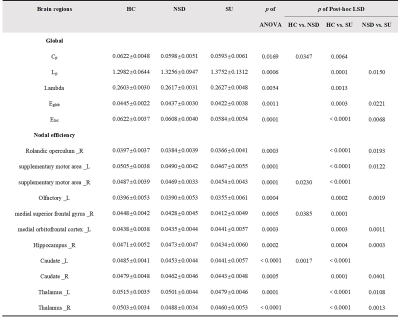
Results
Table 1 showed the demographic and clinical data of all participants. All participants, including HC, NSD and SU groups, showed a small-world architecture (γ > 1, λ ≈ 1, γ/λ > 1) at all connection densities. Eglob and Eloc were decreased in SU group compared both HC and NSD groups; Cp was decreased in NSD and SU groups compared to HC group; Lp was increased in SU group compared both HC and NSD groups; λ was increased in SU group compared to HC group (Table2, Figure1). In addition, among three groups, the nodal degree differed in the fronto-striatum-limbic-thalamic circuit, including bilateral thalamus, caudate, supplementary motor area (SMA), left olfactory, medial orbitofrontal cortex (mOFC), and right hippocampus, medial superior frontal gyrus (mSFG), Rolandic operculum (ROL)(Table2, Figure2, p < 0.05, Bonferroni corrected). The post-hoc t-tests results showed in Table2. We found that both HAMD and HAMA scores were negatively correlated with the nodal efficiency of right SMA (Figure2).Discussion
The human brain network is generally organized as a small-worldness network that features by highly efficient segregated processing and highly integrated information processing [6]. In the SU group, the decreased Eglob and increased Lp/λ showed weaker integration, while the decreased Eloc and Cp showed decreased segregation in the brain structural network. Weaker small-worldness is defined as decreased segregation and weaker integration, which is a pattern among altered small-world properties [7]. Our study showed a shift toward weaker small-worldness of global topological alterations in MDD patients with suicidality compared to HC and PC groups.Apart from the above global topological properties, we found the SU group showed decreased nodal efficiency in the fronto-striatal-limbic-thalamic circuit. The abnormalities of fronto-striatal-limbic-thalamic circuit in suicidal patients were reported in many neuroimaging studies [8-11]. The decreased nodal efficiency involved these circuits, suggesting impaired cognitive control and emotional regulation immediately preceding suicidal actions in MDD patients.
Conclusion
In summary, the structural connectome showed a weaker small-worldness and altered nodal efficiency in the fronto-striatal-limbic-thalamic circuit in depressed patients with suicidality. These alterations of topological organization in depressed suicidal brain structural network provides insights into the underlying neurobiology of suicidal brain.Acknowledgements
We thank the patients and volunteers for participating in this study. This study was supported by the National Natural Science Foundation (Grant Nos. 81971595, 81771812, 81761128023 and 81621003),the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2020HXFH005) and the Department of Science and Technology of Sichuan Province (No. 2020YFS0118).References
1. (WHO)., W.H.O. Suicide statistics. . 2016.
2. Jelescu, I.O., et al., In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. Neuroimage, 2016; 132:104-114. 3. Jenkinson, M., et al., FSL. NeuroImage, 2012; 62:782-790.
4. Maslov, S. and K. Sneppen, Specificity and stability in topology of protein networks. Science, 2002; 296:910-3.
5. Achard, S. and E. Bullmore, Efficiency and cost of economical brain functional networks. PLoS Comput Biol, 2007; 3:e17.
6. Bullmore, E. and O. Sporns, The economy of brain network organization. Nat Rev Neurosci, 2012; 13:336-49.
7. Suo, X., et al., Psychoradiological patterns of small-world properties and a systematic review of connectome studies of patients with 6 major psychiatric disorders. J Psychiatry Neurosci, 2018; 43:170214.
8. Wagner, G., et al., Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage, 2011; 54:1607-14.
9. Fan, S., et al., Gray and white matter differences in adolescents and young adults with prior suicide attempts across bipolar and major depressive disorders. Journal of Affective Disorders, 2019; 245:1089-1097.
10. Monkul, E.S., et al., Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Molecular Psychiatry, 2007; 12:360-366.
11. Jia, Z., et al., Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci, 2014; 39:170-7.
Figures