1659
Morphological changes of the corpus callosum in antipsychotic-naive first-episode schizophrenia before and 1-year after treatment1Huaxi MR Research Center (HMRRC), Functional and molecular imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati, Cincinnati, OH, United States
Synopsis
The present study demonstrates deficits of the callosal morphology in schizophrenia, which may reflect an neurodevelopment aberration. And short-term antipsychotics may not have a significant impact on the CC morphology in the early stage of illness.
Introduction
The corpus callosum (CC) is known to be altered in patients with chronic schizophrenia[1-3]. However, its morphologic characteristics are less well studied in treatment-naive first-episode schizophrenia patients, as is the effect of antipsychotic treatment on this structure. Hence, we recruited a large sample of antipsychotic-naive first-episode schizophrenia patients (AN-FES) to explore callosal morphology. We also tested for changes of the CC morphology over the first year of illness in a subgroup of patient available for follow-up at 1-year.Methods:
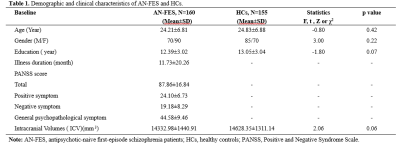
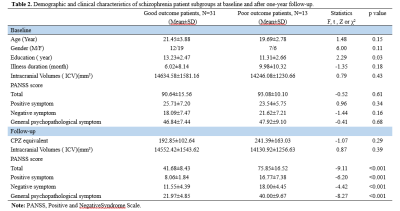
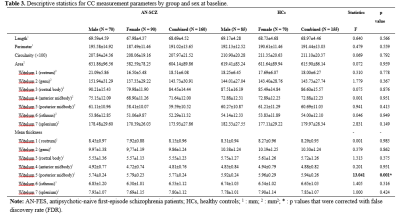
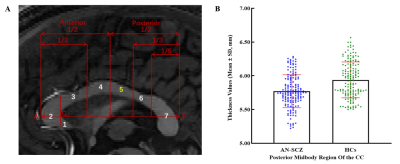
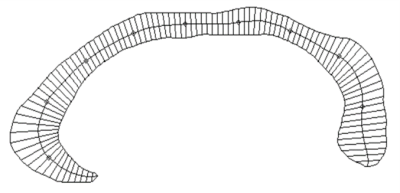
High resolution T1-weighted images and the Positive and Negative Symptom Scale (PANSS) scores were acquired from one hundred and sixty AN-FES and 155 healthy controls (HCs) before treatment initiation. Among the patients, forty-four were available for follow-up studies after one year of antipsychotic treatment, and were divided into good-outcome (n = 31) and poor-outcome subgroups (n = 13) based on whether there was a 50% reduction percent of total PANSS scores from baseline. The Yuki module of automated registration toolbox (ART) was used to automatically identify the mid-sagittal plane (MSP) and obtain morphological measurement parameters of the CC of each participant, including total CC area, perimeter, length, thickness, circularity and the area of seven CC subregions delineated by Witelson[4-6] (Fig. 1A). The 7 subregions included the rostrum (W1), genu (W2), rostral body (W3), anterior midbody (W4), posterior midbody (W5), isthmus (W6), and splenium (W7). The CC thickness profile consisted of 99 non-zero thickness values at equally spaced intervals along the anterior-posterior midline of the CC (Fig. 2). In addition, the 99 thickness values were assigned to the 7 Witelson subregions: Witelson 1 (lines 2–6), Witelson 2 (lines 7–20), Witelson 3 (lines 21–33) Witelson 4 (lines 34–50), Witelson 5 (lines 51–66), Witelson 6 (lines 67–79) and Witelson 7 (lines 80–100). Averages for each subregion were determined. For subregional values of the CC at baseline, we applied multivariate analysis of covariance (MANCOVA) to test for overall between-group differences. Univariate ANCOVA were conducted to test for baseline group differences in total area, perimeter, length and circularity. We also examined correlations of clinical symptoms with statistically significant group differences in callosal metrics. For longitudinal analyses, a repeated measures ANOVA was performed to test for change in CC. Statistical significance was set at p<0.05 two-tailed and p-values were corrected for multiple comparisons using the false discovery rate (FDR) procedure.Results:
Compared with HCs, AN-FES patients showed a significant reduction of thickness in the posterior midbody of the CC (Fig. 1B). This deficit was correlated with severity of negative symptoms. The averaged thickness values of the other six subregions were not significantly different. In addition, ANCOVAs showed no significant difference in the total area or seven subregional areas of the CC in the MSP between AN-FES and HCs. There was also no significant group difference in other metrics including perimeter, length and circularity between the two groups. After one year of antipsychotic treatment, there was no significant change in CC morphology in schizophrenia patients. There was no significant difference of CC morphology between good-outcome and poor-outcome subgroups at baseline, or at 1-year follow-up.Discussion:
Our finding showing a significant and stable deficit of CC morphology in schizophrenia is consistent with the hypothesis of a neurodevelopmental aberration evident in the early stage of illness [7]. Callosal thickness reflects the number of white matter fibers or the degree of myelination. Previous studies indicate that the reduced thickness of CC in schizophrenia mainly represents a loss of myelination in adolescence and early adulthood [8-10]. It is well known that the callosum rapidly develops during adolescence and early adulthood, and the developmental cycle of the posterior region is shorter than that of the anterior region [11-13]. Our findings in FES patients and other data suggest development deficits of the CC in schizophrenia that occur prior to the onset of illness [14]. Thus, our finding of a reduced thickness of the posterior midbody of the CC in AN-FES provides evidence to support an interruption or alteration of the developmental trajectory of CC myelination. The current study, which followed a subgroup of patients 1 year after baseline testing, showed no significant changes of CC morphology in schizophrenia after one year of antipsychotic treatment. We also did not observe significant differences in CC morphology between poor-outcome and good-outcome patients. Thus, our observation of posterior midbody changes were related to negative symptom severity, but not to treatment response. While previous studies found that long-term antipsychotic treatment might change the CC morphology in schizophrenia and good-outcome patients revealed a less pronounced decline in size compared to poor-outcome patients [15,16], these findings were not apparent in our FES sample. Some neuroimaging studies suggest progressive CC deficits over the longer-term course of illness in schizophrenia, which may account for why in our FES sample we did not detect these effects [17,18].Conclusions:
Thickness of the posterior midbody of the CC is reduced early in the course of schizophrenia before treatment. This alteration was not affected by antipsychotic treatment and was unrelated to drug treatment response.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 81671664 to S.L., 81820108018 to Q.G and J.A.S., 81901705 to Y.X.), grants from the Humboldt Foundation (Lui, Sweeney, Xiao), China Postdoctoral Science Foundation (2019M663513), the Postdoctoral Interdisciplinary Research Project of Sichuan University, Sichuan Science and Technology Program (20ZDYF2657), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project No. ZYYC08001, ZYJC18020 to S.L.).References
1. Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. Journal of neurology, neurosurgery, and psychiatry 1995, 58(4):457-461.
2. Arnone D, McIntosh AM, Tan GM, et al. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res 2008, 101(1-3):124-132.
3. John JP, Shakeel MK, Jain S. Corpus callosal area differences and gender dimorphism in neuroleptic-naive, recent-onset schizophrenia and healthy control subjects. Schizophr Res 2008, 103(1-3):11-21.
4. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain : a journal of neurology 1989, 112 ( Pt 3):799-835.
5. Ardekani BA, Bachman AH. Model-based automatic detection of the anterior and posterior commissures on MRI scans. Neuroimage 2009, 46(3):677-682.
6. Ardekani BA, Kershaw J, Braun M, et al. Automatic detection of the mid-sagittal plane in 3-D brain images. IEEE Trans Med Imaging 1997, 16(6):947-952.
7. Venkatasubramanian G, Jayakumar PN, Reddy VV, et al. Corpus callosum deficits in antipsychotic-naive schizophrenia: evidence for neurodevelopmental pathogenesis. Psychiatry Res 2010, 182(2):141-145.
8. Andronikou S, Pillay T, Gabuza L, et al. Corpus callosum thickness in children: an MR pattern-recognition approach on the midsagittal image. Pediatr Radiol 2015, 45(2):258-272.
9. Luders E, Narr KL, Hamilton LS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry 2009, 65(1):84-88.
10. Luders E, Narr KL, Bilder RM, et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage 2007, 37(4):1457-1464.
11. Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthyhuman brain. J Neurosci 2010, 30(33):10985-10990.
12. Keshavan MS, Diwadkar VA, DeBellis M, et al. Development of the corpus callosum in childhood, adolescence and early adulthood. Life sciences 2002, 70(16):1909-1922.
13. Giedd JN, Blumenthal J, Jeffries NO, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry 1999, 23(4):571-588.
14. Balevich EC, Haznedar MM, Wang E, et al. Corpus callosum size and diffusion tensor anisotropy in adolescents and adults with schizophrenia. Psychiatry Res 2015, 231(3):244-251.
15. de Moura MTM, Zanetti MV, Duran FLS, et al. Corpus callosum volumes in the 5years following the first-episode of schizophrenia: Effects of antipsychotics, chronicity and maturation. Neuroimage Clin 2018, 18:932-942.
16. Mitelman SA, Nikiforova YK, Canfield EL, et al. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophr Res 2009, 114(1-3):144-153.
17. Collinson SL, Gan SC, Woon PS, et al. Corpus callosum morphology in first-episode and chronic schizophrenia: combined magnetic resonance and diffusion tensor imaging study of Chinese Singaporean patients. Br J Psychiatry 2014, 204(1):55-60.
18. Walterfang M, Wood AG, Reutens DC, et al. Morphology of the corpus callosum at different stages of schizophrenia: cross-sectional study in first-episode and chronic illness. Br J Psychiatry 2008, 192(6):429-434.
Figures