1658
Association of anterior cingulate glutathione and degree of depression in unmedicated bipolar disorder – a 7T study1Cleveland Clinic Foundation, CLEVELAND, OH, United States
Synopsis
Oxidative stress is a contributing factor in bipolar disorder (BD). Glutathione (GSH) acts as an antioxidant and reduces oxidative stress. GSH level at anterior cingulate cortex was measured in unmedicated patients with BD in depressed state and healthy controls at 7T using semi-LASER sequence. No significant difference in GSH level was observed; however, degree of depression was inversely correlated with GSH level.
INTRODUCTION
Bipolar disorder (BD) is a condition in which patients have periods of depression followed by periods of uncharacteristically elevated mood.1,2 Cellular oxidative stress has been linked to psychopathology of BD in animal models.3,4 GSH reduces reactive oxygen species (ROS) and thus acts as antioxidant;5 reduction in glutathione (GSH) results in increase in ROS and potentially increased oxidative stress.3 Thus GSH plays an important role in BD,3 with depletion in plasma GSH being associated with BD.4Anterior cingulate cortex (ACC) is an important region in the pathophysiology of BD,6-8 which makes it a significantly relevant region to study any modulation of GSH. However, no reductions in ACC GSH compared to healthy controls were observed in young people with BD9 and in euthymic BD.10 On the other hand reduced ACC GSH has been associated with alcohol and tobacco use in BD (depressed, manic, euthymic and mixed state).11 But none of these studies explored ACC GSH in medication free BD in depressed state, which can highlight the role of oxidative stress in depressed state of BD – this is explored here in this study by scanning unmedicated patients with BD and healthy controls at 7T.
METHODS
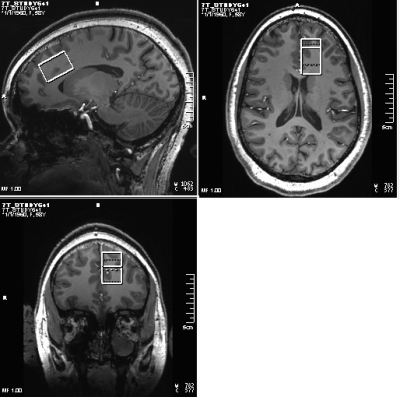
Sixteen patients with BD (Age 30±9y, 3 M, satisfying criteria for Diagnostic and Statistical Manual 5th edition (DSM-V) for BD with current depressive episode, 17-item Hamilton Depression Rating Scale (HAM-D) score >15 and <25, Young Mania Rating Scale (YMRS) < 8, no psychotropic in the last 2 weeks (if previously on fluoxetine then medication free for 5 weeks), no lithium treatment for past 6 months), no alcohol consumption in past 1 week, and 9 healthy controls (29±10y, 3M) were scanned at Siemens 7T Magnetom scanner with a 32-channel receive, single channel transmit head coil under an Institutional Review Board approved protocol. The MRI scans consisted of (i) localizer, (ii) T1-weighted Magnetization Prepared Rapid Acquisition with Gradient Echo (MPRAGE) anatomical scan (TR/TE=2250/2.97ms, matrix=256×256, FOV=204×204mm2) and (iii) semi-LASER (sLASER)12 scan with VAPOR (Variable Power and Optimized Relaxation Delays) for water suppression13 (TR/TE1/TE2/TE3=8000/9/11/9ms, 32 transients) scan of a 20×30×20mm3 voxel at left dorsal/rostral ACC (Fig. 1). In addition, water reference acquisition (with RF off) for eddy current correction and unsuppressed water signal acquisition for quantification were performed. sLASER data were analyzed using MRspa software package (https://www.cmrr.umn.edu/downloads/mrspa/). The analysis consisted of Eddy current, frequency and phase correction, signal averaging and subsequent quantification using LCModel fitting with metabolite concentrations corrected for voxel tissue composition. Voxel segmentation was performed using BET and FAST algorithm14 of FSL software library.15 Mean ACC GSH levels of patients and controls were compared using unpaired t-test. Pearson’s correlation between HAM-D scores and ACC GSH levels was determined.RESULTS and DISCUSSION
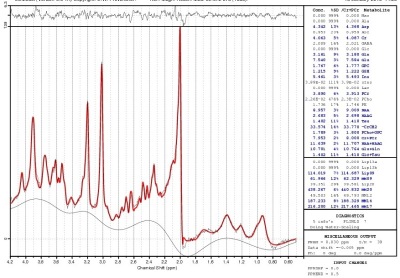
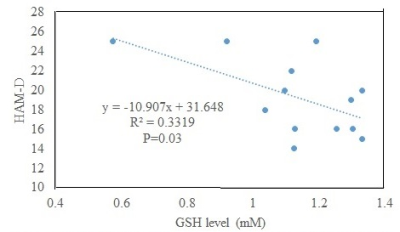
A sample LCModel fitted spectrum is shown in Fig. 2. Data from 3 patients and 1 control were not used because of >20% CRLB of GSH fit. The ACC GSH level in patients (N=13; 1.13±0.21 mM) did not significantly differ from that in controls (N=8; 1.39±0.49 mM); however, the P value (0.10) indicate a tendency towards significance of lower GSH level in patients. The lack of an observed difference in ACC GSH level between patients in depressed state and controls is similar to prior observations in younger patients9 and in patients in euthymic state.10 The observed tendency of lower ACC GSH level in patients could be interpreted to be similar to prior observations of reduced GSH in patients in depressed, manic, euthymic and mixed state with alcohol and tobacco use.11 The patients in the current study, however, were required to be without any alcohol use for at least 1 week prior to the study and were in depressed state. The HAM-D scores were inversely correlated to the ACC GSH level in patients (P=0.03; Fig. 3), i.e. patients with higher GSH exhibited less depression. This behavior is different from absence of correlation seen in the group of younger patients (with 87% of them under some psychotropic medications) that included bipolar spectrum disorder as well.9 In addition, it should be noted that in contrast to the previous studies the current research was performed at 7T with improved reproducibility (and thus reliability) of GSH measurements.16CONCLUSION
ACC GSH level between unmedicated patients with bipolar disorder is not significantly different from that in healthy controls. Degree of depression, as measured by HAM-D score, shows an inverse correlation with GSH level.Acknowledgements
This study was conducted with grant finding support from National Institutes of Mental health (NIMH, R01MH113256(AA)). We thank Sineyob Ahn, Siemens Healthineers, for support with sLASER sequence used in this study.
References
1. Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508.
2. Association AP. Diagnostic and Statistical Manual of Mental Disorders 5th ed: Arlington: American Psychiatric Publishing; 2013.
3. Dean OM, van den Buuse M, Bush AI, et al. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr Med Chem. 2009;16(23):2965-2976.
4. Nucifora LG, Tanaka T, Hayes LN, et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl Psychiatry. 2017;7(8):e1215.
5. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489-492.
6. Sanches M, Amorim E, Mwangi B, Zunta-Soares GB, Soares JC. Smaller left anterior cingulate cortex in non-bipolar relatives of patients with bipolar disorder. Braz J Psychiatry. 2019;41(3):254-256.
7. Jelen LA, King S, Horne CM, Lythgoe DJ, Young AH, Stone JM. Functional magnetic resonance spectroscopy in patients with schizophrenia and bipolar affective disorder: Glutamate dynamics in the anterior cingulate cortex during a working memory task. Eur Neuropsychopharmacol. 2019;29(2):222-234.
8. Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102(4):373-379.
9. Lagopoulos J, Hermens DF, Tobias-Webb J, et al. In vivo glutathione levels in young persons with bipolar disorder: a magnetic resonance spectroscopy study. J Psychiatr Res. 2013;47(3):412-417.
10. Soeiro-de-Souza MG, Pastorello BF, Leite Cda C, Henning A, Moreno RA, Garcia Otaduy MC. Dorsal Anterior Cingulate Lactate and Glutathione Levels in Euthymic Bipolar I Disorder: 1H-MRS Study. Int J Neuropsychopharmacol. 2016;19(8).
11. Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. The impact of alcohol and tobacco use on in vivo glutathione in youth with bipolar disorder: an exploratory study. J Psychiatr Res. 2014;55:59-67.
12. Scheenen TW, Heerschap A, Klomp DW. Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. MAGMA. 2008;21(1-2):95-101.
13. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649-656.
14. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45-57.
15. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-219.
16. Terpstra M, Cheong I, Lyu T, et al. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med. 2016;76(4):1083-1091.