1657
Investigation of Microstructural Alterations of the Corpus Callosum in Autism Using Multi-Shell Diffusion MRI and Quantitative Relaxometry1Pediatrics, University of Wisconsin–Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin–Madison, Madison, WI, United States, 3Waisman Center, University of Wisconsin–Madison, Madison, WI, United States, 4Radiology, University of Utah, Salt Lake City, UT, United States, 5Neurology, University of Utah, Salt Lake City, UT, United States, 6Psychiatry, University of Utah, Salt Lake City, UT, United States, 7Psychology and Neuroscience Center, Brigham Young University, Provo, UT, United States, 8Neurology, University of California–Davis, Davis, CA, United States, 9Psychiatry, Harvard School of Medicine, Boston, MA, United States, 10Pediatrics, University of Utah, Salt Lake City, UT, United States, 11Psychiatry, University of Wisconsin–Madison, Madison, WI, United States
Synopsis
White matter microstructural alterations are consistently reported and believed to play a significant role in the defining characteristics of autism spectrum disorder. In this work, we utilized advanced diffusion imaging and quantitative relaxometry to examine microstructural differences in autism. We observe significant group and age-related deviations of the corpus callosum, as well as more widespread alterations of the neurite microstructure. These results are consistent with previous findings of the corpus callosum in ASD and suggest that while sensitive to underlying microstructural deviations, advanced diffusion imaging and relaxometry provide complementary information.
Introduction
White matter microstructural alterations are consistently reported and believed to play a significant role in the defining characteristics of autism spectrum disorder (ASD)1,2. In particular, diffusion tensor imaging (DTI) studies have repeatedly suggested the microstructure of the corpus callosum to be involved in neuropathology of ASD3,4, with overall group and age-related differences of fractional anisotropy and diffusivity alterations observed1. Beyond DTI, advanced diffusion MRI techniques, such as Neurite Orientation Dispersion and Density Imaging (NODDI)5, as well as quantitative relaxometry6,7, may be used to provide sensitive and complementary measurements of tissue microstructure. Such measurements may be additionally informative to understanding white matter microstructural differences in ASD, however, their application in ASD studies have been limited. Using a multi-shell diffusion acquisition to quantify the NODDI neurite index (NDI) and MP2RAGE8 imaging for quantitative T1 (qT1). mapping, we aimed to investigate differences of the corpus callosum microstructure in a large cohort of ASD and typically developing controls (TDC).Methods
Participants included 176 individuals (n=87 ASD) between 12 and 57 years of age who underwent imaging on a Siemens 3T PRISMA scanner using a 64-channel head coil. Diffusion imaging was performed using a four-shell b-value diffusion-weighted EPI sequence with 180 directions (350 s/mm2 [12], 1000 s/mm2 [24], 2000 s/mm2 [48], 3000 s/mm2 [96]) and 7 non-diffusion weighted images. Additional diffusion imaging parameters consisted of: TR=4870 ms; TE=92.40 ms; flip angle=78 degrees; multi-band slice acceleration factor of 3; and whole brain coverage with 1.5 mm isotropic resolution. Further b=0 s/mm2 images were acquired with reverse phase encoding polarity to allow for susceptibility distortion correction9. A sagittal MP2RAGE scan with 1 mm isotropic resolution was additionally acquired with the following scanning parameters: TR=5000 ms; TE=2.93; TI=700 and 2030 ms; and flip angles= 4 and 5 degrees, respectively. Image Processing: Diffusion images were processed using an in-housing processing pipeline utilizing functions from DIPY toolkit10, FMRIB Software Library (FSL)11, and MRtrix12 software packages. Processing included removal of Rician noise13, removal of Gibbs ringing artifact14, distortion correction using FSL’s topup9 and correction for eddy-currents and motion using FSL’s EDDY tool15 with outlier replacement16 enabled. FSL’s ‘eddy-quad’ was used to identify outlier DWI volumes, which were removed before fitting of DWI data17. Diffusion images were subsequently fit the NODDI model to quantify NDI. NDI maps were normalized to a study-specific template using the Advanced Normalization Tools suite18. An open-source package (https://github.com/JosePMarques/MP2RAGE-related-scripts) was used to create qT1 maps from MP2RAGE data. The MP2RAGE T1-weighted images were normalized to the MNI template using ANTs18 and transformations were applied to the qT1 maps. Mean NDI and qT1 values were computed from the corpus callosum regions of the genu, body and splenium from the JHU white matter atlas. Statistical Analyses: Group differences and age-by-group interactions were examined using linear models with R software, controlling for age. Exploratory voxelwise analyses were also performed to examine microstructural differences beyond the corpus callosum using nonparametric permutation testing, adjusting for age and utilizing threshold free clustering approach19.Results
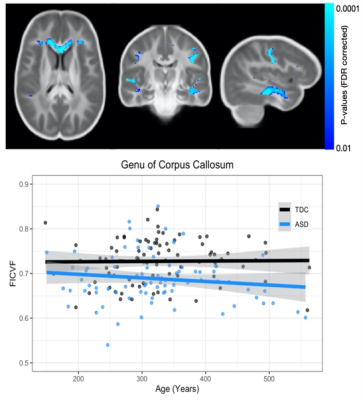
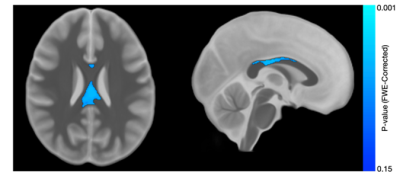
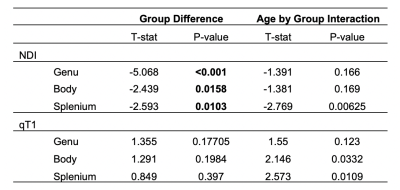
Results from the corpus callosum region of interest analysis are presented in Table 1. Overall, we observed significant group differences in NDI for all subsections of the corpus callosum, such that TDC subjects had significantly greater NDI than ASD subjects. Moreover, NDI of the splenium exhibited a significant age-by-group interaction. For qT1, significant age-related deviations of the body and splenium were found, with increased qT1 in the ASD group. Exploratory voxelwise analysis showed significantly lower NDI in the ASD group across white matter, including the genu of the corpus callosum, superior longitudinal fasciculus, and uncinate fasciculus (p<0.05, corrected; Figure 1). These voxelwise analyses of qT1 were consistent with our ROI based analyses in the body and splenium of the corpus callosum (Figure 2).Discussion
Utilizing advanced diffusion and quantitative relaxometry measures, we observed significant differences and age-related deviations of the corpus callosum microstructure between the ASD and TDC groups. These results are consistent with the findings of DTI studies and continue to suggest significant microstructural alterations of the corpus callosum in ASD. Moreover, while NDI was observed to exhibit more widespread group differences, the age-related deviations of qT1 a were localized in the corpus callosum. This finding may suggest that while sensitive to underlying microstructural deviations, NDI and qT1 provide complementary information. Indeed, qT1 is sensitive to myelin content and therefore our results may suggest that ASD individuals exhibit different patterns of myelination compared to the TDC group, while the widespread NDI differences may suggest greater differences of the neurite microstructure. Future analyses will begin to investigate longitudinal NDI and qT1 measurements, which will provide improved insight into the developmental trajectories of the white matter microstructure and may help identify mechanisms underlying atypical development in ASD.Acknowledgements
We sincerely thank the adolescents and their families who participated in this research. This work was supported by grants R01 MH097464 (Dr. Lainhart), R01 MH080826 (Dr. Lainhart), and R00 MH11056 (Dr. Dean) from the National Institute of Mental Health, National Institutes of Health. Infrastructure support was also provided, in part, by grant U54 HD090256 from the Eunice Kennedy Shriver NICHD, National Institutes of Health (Waisman Center).References
1 Travers, B. G. et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 5, 289-313, doi:10.1002/aur.1243 (2012).
2 Ameis, S. H. & Catani, M. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex 62, 158-181, doi:10.1016/j.cortex.2014.10.014 (2015).
3 Travers, B. G. et al. Atypical development of white matter microstructure of the corpus callosum in males with autism: a longitudinal investigation. Mol Autism 6, 15, doi:10.1186/s13229-015-0001-8 (2015).
4 Alexander, A. L. et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage 34, 61-73, doi:10.1016/j.neuroimage.2006.08.032 (2007).
5 Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000-1016, doi:10.1016/j.neuroimage.2012.03.072 (2012).
6 Deoni, S. C. Quantitative relaxometry of the brain. Top Magn Reson Imaging 21, 101-113, doi:10.1097/RMR.0b013e31821e56d8 (2010).
7 Alexander, A. L. et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 1, 423-446, doi:10.1089/brain.2011.0071 (2011).
8 Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49, 1271-1281, doi:10.1016/j.neuroimage.2009.10.002 (2010).
9 Andersson, J. L., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870-888, doi:10.1016/S1053-8119(03)00336-7 (2003).
10 Garyfallidis, E. et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 8, 8, doi:10.3389/fninf.2014.00008 (2014).
11 Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. Fsl. Neuroimage 62, 782-790, doi:10.1016/j.neuroimage.2011.09.015 (2012).
12 Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137, doi:https://doi.org/10.1016/j.neuroimage.2019.116137 (2019).
13 Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394-406, doi:10.1016/j.neuroimage.2016.08.016 (2016).
14 Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76, 1574-1581, doi:10.1002/mrm.26054 (2016).
15 Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063-1078, doi:10.1016/j.neuroimage.2015.10.019 (2016).
16 Andersson, J. L. R., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141, 556-572, doi:10.1016/j.neuroimage.2016.06.058 (2016).
17 Bastiani, M. et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage 184, 801-812, doi:10.1016/j.neuroimage.2018.09.073 (2019).
18 Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12, 26-41, doi:10.1016/j.media.2007.06.004 (2008).
19 Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83-98, doi:10.1016/j.neuroimage.2008.03.061 (2009).
Figures