1656
A Multi-Diffusion Model Investigation of White Matter Microstructure in Psychotic Spectrum Disorders1Radiology, New York University School of Medicine, New York, NY, United States, 2New York University School of Medicine, New York, NY, United States
Synopsis
We employed the recent Bingham neurite orientation dispersion and density imaging (NODDI-B) and diffusion kurtosis imaging (DKI) techniques, alongside classical diffusion tensor imaging (DTI) and found that psychotic spectrum disorders (PSD) and PSD sub-types had significantly increased mean and radial diffusion and orientation dispersion (MD, RD, ODI, ODIs) and significantly decreased mean and radial kurtosis (MK, RK), fractional anisotropy (FA) and neurite density index (NDI) in sub-cortical WM ROIs compared to healthy controls (HC). We also found significant relationships across PSD and HC groups between WM dMRI metrics and tests of episodic and working memory and several schizotypal traits.
Background
Diffusion tensor imaging (DTI)-based white matter (WM) abnormalities have been a long-standing finding in Psychotic Spectrum Disorders (PSD)1. DTI metrics have been used as a biomarker to predict treatment2, track symptom progression3 and improve patient classification4. However, the underlying nature of DTI changes remains unclear, due to the method’s lack of specificity5. Previous histological studies have suggested an array of microstructural abnormalities that may underlie diffusion changes in PSD including altered myelinated fibers6 and ogliodendrocytes7, reduced cellular density8, protein accumulation9 and increased inflammatory cells and edema10. The aim of this research was to evaluate if the recent Bingham neurite orientation dispersion and density imaging (NODDI-B) and diffusion kurtosis imaging (DKI) techniques, alongside classical DTI can describe sub-cortical WM microstructural changes in PSD and their association with cognition and symptoms. NODDI-B modifies the original NODDI model to better characterize the anisotropic orientation dispersion of neurites, which is a prominent feature of WM11. DKI, on the other hand, is an extension of DTI that accounts for the non-Gaussian water diffusion contributions to the diffusion MRI signal and provides both mean and directional kurtosis indices that reflect tissue microstructural complexity12. Mapping the NODDI-B, DKI and DTI-derived measures of WM microstructural complexity in PSD, along with their relationship with cognition and symptoms, may inform upon the biological bases of white matter integrity pathology in PSD and ultimately contribute to development of targeted treatment approaches.Methods
Anatomical and diffusion MRI data were acquired for 54 PSD patients (16 schizophrenia, 20 schizoaffective, 18 bipolar with psychotic features), and 35 healthy comparison controls (HC) (men and women, 18-31 years old) using a 3T Prisma MRI scanner. The NODDI-B model was implemented using DMIPY software to obtain secondary (ODIs), primary (ODIp) and total (ODI) orientation dispersion indexes along with a neurite density index (NDI)11,13. The diffusion and kurtosis tensors were calculated as previously described by Tabesh et al. 2011 and used to derive three-dimensional maps of mean (MD), axial (AD) and radial (RD) diffusion, fractional anisotropy (FA) and mean (MK), radial (RK) and axial (AK) kurtosis metrics for each study participant14,15. Mean WM diffusion metrics were subsequently calculated for the 34 bilateral sub-cortical WM regions of interest (ROI) delineated by the Desikan-Killiany atlas16. Independent t-test analyses and analyses of covariance that accounted for age, sex and pre-morbid IQ were used to evaluate differences in dMRI metrics between PSD, PSD sub-types and HC participants in each ROI. Further analyses used Pearson’s and Spearman’s correlations to test whether dMRI metrics relate to: 1) cognitive function, which was assessed using a battery of working and episodic memory and processing speed neuropsychological tests, and 2) the diagnostic interview for genetic studies (DIGS) schizotypal traits across both PSD and HC groups. The Benjamini-Hochberg (BH) procedure was employed in each analysis to correct for multiple comparisons and decrease the false discovery rate (FDR)17. Differences were considered significant for q < .05 BH FDR and at trend-level for p < .05.Results
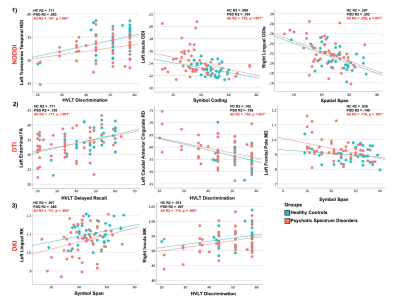
Controlling for age, sex and pre-morbid IQ had no effect on group differences and therefore t-test results are presented here. Significant between-group differences were observed with 1) significantly increased MD, RD, ODI and ODIs, and 2) significantly decreased MK, RK, FA and NDI metrics in sub-cortical WM ROIs in PSD and PSD subtypes. Figures 1 and 2 displays sub-cortical WM regions showing between group differences by projecting them onto the corresponding sub-cortical WM surface. No significant differences were found comparing AD or ODIp metrics between groups. Significant relationships were found across PSD and HC groups between WM dMRI metrics and tests of episodic and working memory (Figure 3). Additionally, significant relationships were found between WM dMRI metrics, across PSD and HC groups, and the DIGS in several schizotypal traits (Figure 4). Correlation analyses revealed that in both PSD and HC groups, increased MD, RD, ODI and ODIs and decreased MK, RK, FA and NDI were related to poor cognition and increased symptoms.Discussion
Classical DTI studies have revealed altered WM integrity in PSD in a number of WM tracts. Here we focus on sub-cortical peripheral WM brain regions delineated through the Desikan-Killiany atlas. Results presented here suggest WM integrity changes in PSD occur in widespread sub-cortical regions and are highly directional, driven by pathology which increases the diffusion and orientation dispersion of neurites and decreases complexity along the secondary diffusion direction, i.e. perpendicular to neurites. Increased RD and ODIs and decreased RK in PSD suggests demyelinated axons or vasogenic edema as a primary pathology18,19. These pathologies also appear to be related to working and learning memory, processing speed and schizotypal traits independent of diagnosis, occurring in both HC and PSD groups.Conclusion
We applied, for the first time, the NODDI-B model to describe sub-cortical WM integrity in a PSD cohort alongside DKI and DTI techniques. WM diffusivity, complexity, orientation and density, especially in the radial direction, appear to affect healthy memory, processing speed and associate with the presence of schizotypal symptoms in both HC and PSD groups.Acknowledgements
This work was supported by the R01 MH108962 National Institute of Mental Health award. We greatly thank all of our participants for their help with this study and Researchmatch for supporting our recruitment efforts.References
1. Karlsgodt, K. H. White Matter Microstructure across the Psychosis Spectrum. Trends in Neurosciences (2020).
2. Klauser, P. et al. N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: a double-blind randomized placebo-controlled trial. Transl. Psychiatry (2018).
3. Saito, J. et al. Can reduced leftward asymmetry of white matter integrity be a marker of transition to psychosis in at-risk mental state? Asian J. Psychiatr. (2020).
4. Andreou, C. & Borgwardt, S. Structural and functional imaging markers for susceptibility to psychosis. Molecular Psychiatry (2020).
5. Alexander, A. L., Lee, J. E., Lazar, M. & Field, A. S. Diffusion Tensor Imaging of the Brain. Neurotherapeutics (2007).
6. Davis, K. L. et al. White Matter Changes in Schizophrenia. Arch. Gen. Psychiatry (2003).
7. Uranova, N. A., Vikhreva, O. V., Rachmanova, V. I. & Orlovskaya, D. D. Ultrastructural Alterations of Myelinated Fibers and Oligodendrocytes in the Prefrontal Cortex in Schizophrenia: A Postmortem Morphometric Study. Schizophr. Res. Treatment (2011).
8. Williams, M. et al. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. (2014).
9. Webster, M. J. et al. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain. Behav. Immun. (2001).
10. Najjar, S. & Pearlman, D. M. Neuroinflammation and white matter pathology in schizophrenia: Systematic review. Schizophrenia Research (2015).
11. Tariq, M., Schneider, T., Alexander, D. C., Gandini Wheeler-Kingshott, C. A. & Zhang, H. Bingham-NODDI: Mapping anisotropic orientation dispersion of neurites using diffusion MRI. Neuroimage (2016).
12. Jensen, J. H. & Helpern, J. A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in Biomedicine 23, 698–710 (2010).
13. Fick, R. H. J., Wassermann, D. & Deriche, R. The Dmipy Toolbox: Diffusion MRI Multi-Compartment Modeling and Microstructure Recovery Made Easy. Front. Neuroinform. (2019).
14. Tabesh, A., Jensen, J. H., Ardekani, B. A. & Helpern, J. A. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 65, 823–836 (2011).
15. Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. Fsl. Neuroimage 62, 782–790 (2012).
16. Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
17. Hochberg, B. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 57, 289–300 (1995).
18. Winklewski, P. J. et al. Understanding the physiopathology behind axial and radial diffusivity changes-what do we Know? Frontiers in Neurology (2018).
19. Guglielmetti, C. et al. Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. Neuroimage (2016).
Figures