1655
How neurotransmitter concentrations in default-mode network modulate brain functional activities and connectivities in psychosis1McLean Hospital; Harvard Medical School, Belmont, MA, United States
Synopsis
Failure to suppress default-mode network (DMN) activity during tasks and reduced anti-correlations between DMN and other brain networks at rest has been observed in various psychiatric disorders. However, the molecular mechanisms underlying this phenomenon are poorly understood. It has been shown that the neurotransmitter concentrations in DMN modulate the brain functional activities and connectivities in the healthy brain. In the current study, it was observed that the relationship between DMN neurotransmitter concentrations and the activities of brain functional network breaks down in first-episode psychosis patients. This finding provides opportunities for developing novel treatment strategies and earlier interventions for psychosis.
Introduction
Schizophrenia is a severe mental disorder characterized by hallucinations, disorganized thought, impaired emotional and motivational processes, and cognitive dysfunction. Approximately 1.1% of the population over the age of 18 suffers from this condition. Researchers have sought to identify biomarkers of brain and behavior disorders for the past two decades, yet they are still in the very initial stages. However, the recent development of novel neuroimaging techniques hold promise for the identification of biomarkers based on brain function, structure, and metabolites. One of the most reliable observations from neuroimaging studies, deactivation of the human brain’s default mode network (DMN)(1) is regarded as suppression of internal activity to support external task-related processes. Failure to suppress DMN activity during tasks and reduced anti-correlation between DMN and other brain networks at rest has been observed in various psychiatric disorders (2,3). However, the cellular and molecular mechanisms underlying this phenomenon are poorly understood. At the cellular level, neuronal activity is regulated by multiple neurochemical processes including cycling of glutamate (Glu) and GABA, the major excitatory and inhibitory neurotransmitters in the central nervous system. There has been considerable interest in the role of glutamatergic neurotransmission in the pathophysiology of schizophrenia (4). Determining the nature of glutamatergic abnormalities in schizophrenia may prove useful in understanding symptomatology and in developing novel treatment strategies. By combining functional MRI (fMRI) and magnetic resonance spectroscopy (MRS) techniques, it has been shown how regional neurotransmitters modulate brain functional activity in a series of previous publications (5-7). Particularly, in a recent paper published in Cerebral Cortex (6), we measured the static neurotransmitters in the medial prefrontal cortex (MPFC) and dorsolateral prefrontal cortex (DLPFC) and their relationship with the brain activation/deactivation during the Sternberg working memory task as well as the internetwork connectivity between DMN and the control network (CN) during resting-state and task activation. In the current study, we extended previous research to first episode (FE) schizophrenia patients and their relatives. Working with FE SZ patients, we can obviate any medication effects and the toxic effects of chronic psychosis. MPFC Glu and GABA concentrations, as well as the resting-state functional connectivity were measured. The relationship between the neurotransmitters and the functional connectivity between DMN and CN was also examined.Methods
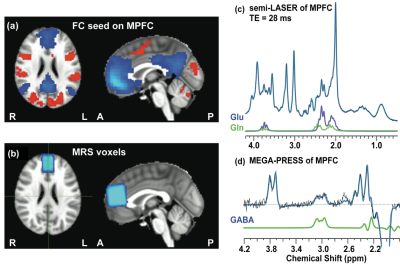
57 subjects, including 20 schizophrenia patients (SZ), 17 relatives (RL) and 20 healthy controls (HC), were recruited in the current study. The MRI/MRS protocols was applied on a Siemens 3 T Trio scanner using a 32-channel volume coil: a high-resolution anatomical scan (5 min), resting-state fMRI (6 min x 2 runs), followed by MRS (20 min for localized shimming, MEGA-PRESS and semi-LASER). Single-voxel proton MRS was acquired at MPFC with a voxel size 30 × 20 × 30 mm3. A modified version of MEGA-PRESS optimized in house for GABA detection was acquired with the following parameters: TE/TR = 68/3000 ms and total 192 averages (scan time=10 min); the editing pulses applied alternatively at frequency of 1.9 or 7.5 ppm interleaved with the averages. A semi-LASER sequence with optimized TE = 28 ms and TR = 3 s and 64 averages (scan time = 4 min) was used to measure Glu and Gln from the same regions as MEGA-PRESS. Fastmap shimming was performed before MRS scans to ensure the full widths at half maxima (FWHM) of water resonance <12 Hz. Spectra with unsuppressed water was also acquired for eddy-current correction and quantification reference. The quantification was performed using LCModel. Resting-state fMRI was collected using a single-shot gradient-echo echo-planar imaging sequence. The imaging parameters were: TR/TE = 2500/30 ms; FA = 78°; slice thickness/gap = 4/0 mm; 39 slices; FOV = 220 × 220 mm2 (3.44 × 3.44 mm2 in-plane resolution). The anatomical data was acquired for structural reference using a MP-RAGE sequence (256 × 256 × 122 matrix size; 1 × 1 × 1.28 mm3 spatial resolution; TI/TR/TE = 1100/1600/2.25 ms; flip angle = 12°). The fMRI was processed in the DPARSF software. The seed region of functional connectivity, MRS voxel location, and representative spectra of semi-LASER and MEGA-PRESS were presented in Figure 1.Results and discussions
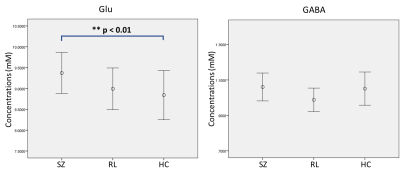
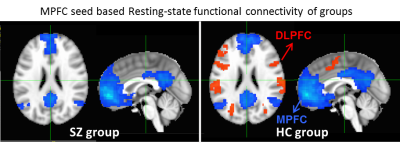
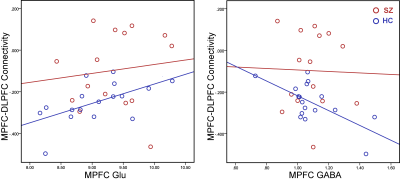
MPFC Glu and GABA concentrations, as well as the resting-state functional connectivity between MPFC and DLPFC were measured. Elevated Glu level in the MPFC in SZ was found, but there was no significant change in GABA (Figure 2). A significant reduction in the functional anti-correlation connectivity between MPFC and DLPFC was also observed in SZ (Figure 3). These findings were consistent with previous reports (8-10). Furthermore, it was observed for the first time that the relationship between MPFC neurotransmitter concentrations and the functional anti-correlation that is seen in healthy people was compromised in first-episode psychosis patients (Figure 4). Coordinated activity within and differential activity between DMN and CN is a critical feature of brain organization. These findings imply that DMN glutamatergic neurotransmission dysfunction has an adverse impact on the interaction between DMN and CN, and it would be very interesting to better understand this dysregulation. With novel insights into neurotransmitters abnormalities and network activities, it provides opportunities for developing novel treatment strategies and earlier interventions for psychosis.Acknowledgements
This work was supported by: K24MH104449 (DO), R01MH114982 (FD, DO)References
1. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98(2):676-682.
2. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A 2009;106(4):1279-1284.
3. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012;16(12):584-592.
4. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2012;37(1):4-15.
5. Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013;33(47):18566-18573.
6. Chen X, Fan X, Hu Y, Zuo C, Whitfield-Gabrieli S, Holt D, Gong Q, Yang Y, Pizzagalli DA, Du F, Ongur D. Regional GABA Concentrations Modulate Inter-network Resting-state Functional Connectivity. Cerebral cortex 2018.
7. Gu H, Hu Y, Chen X, He Y, Yang Y. Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage 2018;185:388-397.
8. Egerton A, Modinos G, Ferrera D, McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry 2017;7(6):e1147.
9. Kim SY, Kaufman MJ, Cohen BM, Jensen JE, Coyle JT, Du F, Ongur D. In Vivo Brain Glycine and Glutamate Concentrations in Patients With First-Episode Psychosis Measured by Echo Time-Averaged Proton Magnetic Resonance Spectroscopy at 4T. Biological psychiatry 2017.
10. Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2011;36(10):2009-2017.
Figures