1647
High resolution magnetic resonance imaging-based texture analysis for the assessment of carotid atherosclerotic plaques vulnerability1Radiology, Renmin Hospital of Wuhan University and Hubei General Hospital, Wuhan, China, 2Advanced Application Team, GE Healthcare, Wuhan, China
Synopsis
This study aimed to assess the vulnerability of carotid atherosclerotic plaques using the combination of radiomics nomogram and clinical high-risk factors. Texture features were extracted from high-resolution multi-contrast magnetic resonance imaging. Radscore was applied to build a diagnostic model with the consideration of hrMRI texture features and patient demography to assess the power in differentiating vulnerable and stable lesions. The radscore showed a better diagnostic performance. The combination model of texture and clinical information had the best performance in assessing lesion vulnerability. This study demonstrated that hrMRI texture features provided incremental value for the carotid atherosclerotic vulnerability assessment.
Introduction and purpose
Carotid atherosclerotic plaque contributes to ~20% of ischemic cerebrovascular events, including transient ischemic attack (TIA) [1]. Clinical trials demonstrated that ultrasonography-defined luminal stenosis ≥70% was predictive for future ischemic event for both symptomatic [2] and asymptomatic [3] patients. However, numerous studies have demonstrated serious limitations of angiology-defined degree of luminal stenosis and carotid ultrasound screening in the general population is therefore not recommended [4]. However, atherosclerosis is a complex structure, e.g., there is existence of mixture of fibrous tissue and lipid [5], different type of collagen (type I and type III) [6]. The complexity in compositional feature might be captured by in vivo imaging showing special image texture that has been least studied. Pilot studies have demonstrated the clinical potential of image texture analysis. This study aimed to build an effective model with the combination of high resolution, multi-contrast magnetic resonance imaging texture features and patient clinical risk factors with the hope to improve the accuracy in differentiating stable and unstable carotid atherosclerotic lesions.Materials and Methods
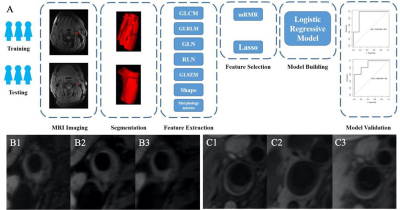
Patients: Fifty patients visited Renmin Hospital of Wuhan University from July 2015 to April 2020 were recruited. All patients had at least 60% carotid luminal stenosis as defined by ultrasound angiography. Imaging: The hrMRI was performed a 3.0T MR750 system (GE Healthcare, USA) with an 8-channel carotid coil (GE Healthcare, USA). The protocol of hrMRI included, 2D T1-weighted double inversion recovery FSE, proton density (PD) weighted FSE, 2D T2-weighted double inversion recovery FSE. Data processing: hrMRI was segmented manually using ITK-SNAP (www.itksnap.org). Each lesion was classified as an AHA type according to the modified scheme proposed by Cai et al [7]. AHA type IV-V and VI were characterized as vulnerable plaque. Type IV-V: plaque with a lipid or necrotic core surrounded by fibrous tissue with possible calcification; Type VI: complex plaque with possible surface defect, hemorrhage or thrombus. Radiomics features were extracted using the Analysis Kit Software 3.1.0 (GE Healthcare, China). Max-Relevance and Min-Redundancy (mRMR) and Least Absolute Shrinkage and Selection Operator (LASSO) were employed for an optimized model. Radscore was applied to build a diagnostic model with the consideration of hrMRI texture features and patient demography to assess the power in differentiating vulnerable and stable lesions. The statistics were carried out using R 3.6.1 (http://www.Rproject.org) (Fig. 1).Results
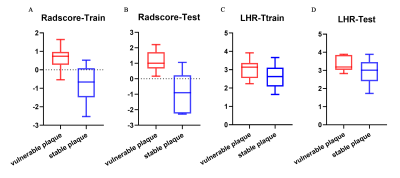
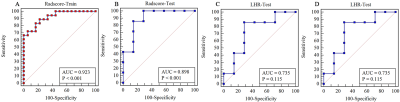
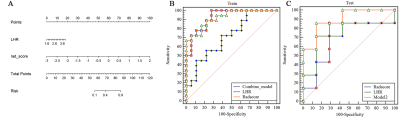
hrMRI from 50 patients were used for the final analyses (age, 54.0±12.9 year; BMI, 23.63±2.24 kg/cm2). Twenty-five plaques were stable and 25 unstable. The lesions were randomly distributed as training and testing groups in a ratio of 7:3. Two hundred and eighty radiomic features were initially extracted from T1 and T2-weighted images. Although the radscore for the T1-weighting could differentiate stable and unstable lesions in the training group (p=0.021), but not for the testing group (p=0.79) with AUC being 0.72 (95% confidence interval (CI), 0.56-0.89) in training group and 0.45 (95% CI, 0.13-0.77) in testing group. Radscore based on T2-weighted image of vulnerable plaque was significantly higher than the one of stable plaque in both training (p<0.0001) and testing groups (p=0.011) (Fig 2). The AUC was 0.923 [95% CI, 0.784-0.985] in the training group and 0.898 (95% CI, 0.622-0.993) in the testing group (Fig 3). Moreover, the LDL/HDL ratio (LHR) of vulnerable plaque was also significantly higher than the one of stable lesion in the training group (p=0.029, Fig 2) with AUC being 0.713(Fig 3). A multivariable logistic regression model with the combination of radscore and LHR was constructed which was visualized by a nomogram (Fig 4). The AUC of the combined model was 0.926 (95% CI, 0.788-0.986) in the training group and 0.898 (95% CI, 0.622–0.993) in the testing group.Discussion and Conclusions
This study demonstrated that the vulnerability of carotid plaques could be assessed via hrMRI-based radiomics model, constructed by radiomics signature and clinical data. Traditionally, the lesion vulnerability was assessed according to geometric parameters, such as stenosis [8], the size of lipid-rich necrotic core, and thickness of fibrous cap, and presence of inflammation and IPH. In addition to hrMRI, we chose age, sex, LDL, HDL and LHR as clinical factors to predict plaque type as they are proved high-risk factors for cardiovascular diseases [9]. LHR didn’t have a better performance than the radiomics model in both training (AUC, 0.713 vs. 0.923) and testing groups (AUC, 0.735 vs. 0.898). However, the combination of LHR and radiomics could better predict the plaque type than any of them alone. In conclusion, we built a novel model combining the radiomics features and clinical risk factors for the prediction of carotid atherosclerotic plaque vulnerability. Despite of interesting findings, limitations exist. Firstly, the patient sample size was small. Secondly, radiomics and LHR were considered in our model without any other high-risk clinical features such as carotid plaque stenosis, lipid-rich necrotic core, and fibrous cap, etc.Acknowledgements
Funding: This project was supported by the the Fundamental Research Funds for the Central Universities (NO.2042018kf0130)References
- J. F. Fairhead, Peter M. Rothwell (2005). The Need for Urgency in identification and treatment of symptomatic carotid stenosis is already established. Cerebrovasc Dis 19: 355-358.
- Barrnelt HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox, AJ, et al. North American Symtomatic Carotid Endarterectomy Trial Collaborators (1991) Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 235: 445-453.
- LeFevre ML, U.S. Preventive Serviced Task Force (2014) Screening for asymptomatic carotid artery stenosis: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 5: 356-362.
- Rothwell P M, Gibson R, Warlow CP (2000) On behalf of the European Carotid Surgery Trialists, Collaborative Group. Interrelation between plaque surface Morphology and degree of stenosis on carotid angiograms and the risk of Ischemic stroke in patients with symptomatic carotid stenosis. Stroke 31: 615–621.
- Van Dijk AC, Truijman MT, Hussain B, Zadi T, Saiedie G, de Rotte AA, Liem MI, et al (2015) Intraplaque hemorrhage and the plaque surface in carotid atherosclerosis: the plaque at RISK study (PARISK). AJNR Am J Neuroradio 36: 2127-2133.
- Kolossvary M, Karady J, Kikuchi Y, Ivanov A, Schlett CL, LU MT, Foldyna B, et al (2019) Radiomics versus Visual and Histogram-based Assessment to Identify Atheromatous Lesions at Coronary CT Angiography: An ex Vivo Study. Radiology 93: 89-96.
- Hatsukami TS, Yuan C (2010) MRI in the early identification and classification of high-risk atherosclerotic carotid plaques. Imaging Med 2: 63-75.
- Ju K, Zhong L, Ni X, Cao H, Cheng G, Ding L (2018) Cerebral vasomotor reactivity predicts the development of acute stroke in patients with internal carotid artery stenosis. Neurol Neurochir Pol 52: 374-378.
- Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D, Wasserman BA, et al (2019) Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 18: 559-572.
Figures