1646
Removing pulsatile flow artifacts in 2D TOF MRA with Temporal Harmonic Encoding1Department of Radiology, Mayo Clinic, Rochester, MN, United States, 2Philips Healthcare, Gainesville, FL, United States
Synopsis
2D TOF MRA provides important diagnostic information around carotid bifurcation. However, the image is prone to artifacts caused by pulsatile flow. The present work utilized Temporal Harmonic Encoding scheme to reduce these artifacts by extracting only the static signals for MRA. Meanwhile, the harmonic components containing dynamic information could also be used as a reference.
Introduction
Flow related enhancement in MRI is one of the principal endogenous contrasts to image vasculature. A 2D Time of Flight MR angiography scan is commonly used to exam the carotid bifurcation1,2. As the contrast relies on the unsaturated proton in the inflow, pulsation from cardiac motion sometimes induces artifacts in 2D TOF MRA due to signal variations. Recently parallel imaging strategies including additional dimension in reconstruction have shown potential in improving image quality when motion is involved3-5. While these methods mainly emphasize recovering temporal information with accuracy, the present work, Temporal Harmonic Encoding, focuses on removing artifacts in the static component for MRA and makes use of the temporal harmonic component as additional reference.Materials and Methods
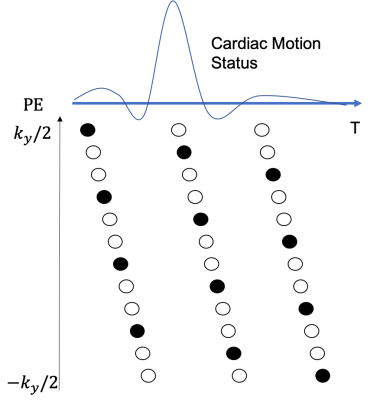
Three healthy volunteers were scanned on a 3.0T scanner (Elition, Philips Healthcare, Best, Netherland) following informed consent approved by the institutional review board. A 2D spoiled gradient echo sequence having flip angle = $$$ 60^0$$$ , TR = 16.0 ms and TE = 2.0 ms was utilized to image $$$ 1 \times 1 mm^2$$$ in-plane resolution and 4mm slice thickness with 2mm slice overlap. Conventional 2D TOF was performed without gating while Temporal Harmonic Encoding was equipped with peripheral gating to synchronize pulsatile motion of the flow. Each cardiac cycle is divided into a designated number of reconstruction phases ($$$R_p$$$) for Temporal Harmonic Encoding. Fig 1. Illustrates an example for a 3-phase scheme ( $$$R_p$$$ = 3). The number of phase encoding lines for each cardiac phase is set to be close to $$$[ \frac{Cardiac\ Cycle}{R_p \times TR} ]$$$ , where [$$$\bullet$$$] is the floor function, leading to the total number of phase encoding lines in a cardiac cycle close to $$$ \frac{Cardiac \ Cycle} {TR}$$$. The acquisition scheme ensures the entire k-space is nearly covered throughout the imaging process. Therefore, the total scan time for Temporal Harmonic Encoding is expected to be slightly larger than the conventional ungated 2D TOF method under the same spatial resolution. An increase around 10-12% of scan time was seen in the experiments, for which the heart rates of the subjects were ranging from 70 to 80 bps.The acquired data were then retrospectively gridded to the designated cardiac phases for reconstruction. The reconstruction procedure can be described as $$ min_{y,f} | s_{k_y,t} - MEC\rho_{y,f}|^2_2 $$, where $$$ s_{k_y,t}$$$ is the acquired k-space data, $$$ \rho_{y,f} $$$ the image represented in y-f space, C the coil sensitivity encoding, E the Fourier Transformation operator, and M the sampling mask after retrospective gating. Note that the sampled point in M may not necessarily be on the grid or equally spaced, the reconstruction relies on an iterative solver, such as conjugate gradient implemented in this work, to reach an optimal solution. The harmonic components in the y-f domain will be demonstrated in comparison with the conventional 2D TOF images.
Results
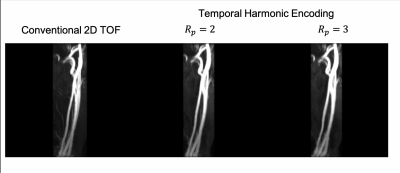
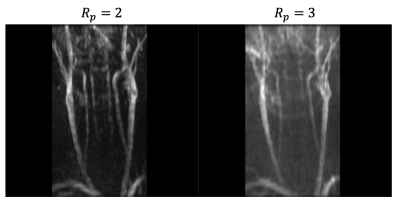
Fig 2. shows the maximal intensity project of the DC component of the Temporal Harmonic Encoding reconstruction along with the conventional 2D TOF MRA. Signal void around bifurcation and inhomogeneous signal distribution in the upstream of common carotid arteries can be found in the conventional scan. These signal drops appears to be mitigated when using Temporal Harmonic Encoding. Fig 3 demonstrates the root-mean-square of the harmonic components. High signal intensity in the harmonic component maps presumably reflects the vessel segments with greater signal changes within the cardiac cycle, perhaps serving as an indicator of local pulsatility.Discussions
Pulsatile flow typically invokes artifacts with inhomogeneous signal drop in the image relying on flow related enhancement contrast. Motion gating has been suggested to smooth these artifacts6. The proposed method further decomposes the harmonic components to remove temporal changing signals to improve image quality. The harmonic components contain the dynamic information as well as mitigating potential motion artifacts. Despite the visible artifacts in the harmonic map, the images may still be used to reveal dynamic contents in the vessel as an additional reference.Conclusion
A Temporal Harmonic Encoding approach is proposed to mitigate artifacts from pulsatile flow in 2D TOF MRA having comparable scan time with the conventional scan.Acknowledgements
This work is supported in part by Philips Healthcare.References
1. Ross, J.S., et al., Multiple reader comparison of 2D TOF, 3D TOF, and CEMRA in screening of the carotid bifurcations: Time to reconsider routine contrast use? PLoS One, 2020. 15(9): p. e0237856.
2. Carriero, A., et al., High-resolution magnetic resonance angiography of the internal carotid artery: 2D vs 3D TOF in stenotic disease. Eur Radiol, 1998. 8(8): p. 1370-2.
3. Breuer, F.A., et al., Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med, 2006. 55(3): p. 549-56.
4. Tsao, J., P. Boesiger, and K.P. Pruessmann, k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med, 2003. 50(5): p. 1031-42.
5. Madore, B., G.H. Glover, and N.J. Pelc, Unaliasing by fourier-encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn Reson Med, 1999. 42(5): p. 813-28.
6. Saloner, D., K. Selby, and C.M. Anderson, MRA studies of arterial stenosis: improvements by diastolic acquisition. Magn Reson Med, 1994. 31(2): p. 196-203.
Figures