1642
Preoperative cerebral small vessel disease is independently associated with cerebral hyperperfusion after carotid endarterectomy1Peking Union Medical College Hospital, Beijing, China, 2GE Healthcare, Beijing, China
Synopsis
Whether preoperative cerebral small vessel disease and carotid near-occlusion are associated with cerebral hyperperfusion (CH) after carotid endarterectomy has not been reported. This study demonstrated that preoperative Fazekas score ≥ 3, number of lacunes ≥ 2 combined with the presence of carotid near-occlusion can accurately predict CH after carotid endarterectomy. This encourages preoperative MRI scans and suggests we should pay particular attention to patients with CSVD and carotid near-occlusion.
Introduction
Cerebral hyperperfusion syndrome(CHS) is a rare but serious complication after carotid revascularization, which often occurs in patients with cerebral hyperperfusion(CH)[1]. Impaired cerebral autoregulation is the most accepted mechanism for CH[2].Given that cerebral autoregulation mainly occurs in the cerebral arterioles and capillaries[2] and that cerebral small vessel disease(CSVD) is strongly linked to impaired cerebral autoregulation[3], we hypothesized that preoperative CSVD is an important risk factor for CH after carotid endarterectomy(CEA). In addition, the association between near-occlusion and CH/CHS in CEA-patients has not been examined.Objectives
To determine whether preoperative CSVD and carotid near-occlusion can predict CH after CEA.Methods
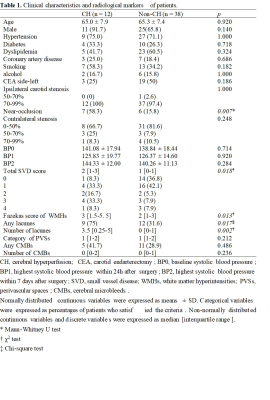
Consecutive patients with carotid stenosis who underwent CEA between May 2015 and September 2020 were included. CH was defined as an increase in cerebral blood flow >100% compared with preoperative values on arterial spin labelling images. The grade or the number of four cerebral small vessel disease markers (white matter hyperintensities(WMHs), lacunes, perivascular spaces, and cerebral microbleeds) were evaluated based on preoperative magnetic resonance imaging. The presence of carotid near-occlusion was defined by computed tomography angiography.Results
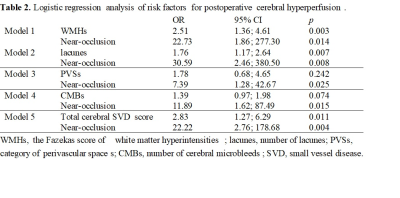
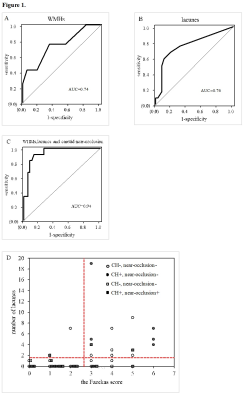
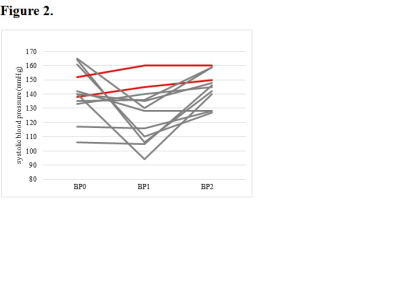
Fifty patients were included. CH after CEA was observed in 12 patients(24%). Logistic regression analysis revealed that white matter hyperintensities(OR=2.46, 95% CI=1.36-4.45; p=.003) and lacunes(OR=1.76, 95% CI 1.17-2.64; p=.007) were significantly associated with postoperative CH. Carotid near-occlusion was associated with CH in all models(p<.01). For the logistic regression model, the combination of a higher Fazekas score(≥3), the number of lacunes ≥2, and the presence of carotid near-occlusion can predict CH after CEA with 91.7% sensitivity and 86.8% specificity. CHS occurred in 2(16.67%) of the 12 patients diagnosed with CH. The maximum systolic blood pressure of the two CHS patients within 24h after surgery was higher than that of the other 10 patients.Conclusion
In patients with carotid stenosis, preoperative WMHs, lacunes, and carotid near-occlusion can predict CH after CEA. Strict control of blood pressure may be important for preventing the progression of CHS.Acknowledgements
We thank Bing Wu from GE Healthcare for the MR technical help.References
1. van Mook WN, Rennenberg RJ, Schurink GW, Van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877–88.
2. Lin YH, Liu HM. Update on cerebral hyperperfusion syndrome. J Neurointerv Surg 2020;12: 788-93.
3. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019;18:684–96.
Figures