1639
Vessel segmentation on intracranial noncontract enhanced dynamic MR angiography using spatial-temporal correlation between voxels1Fudan University, Shanghai, China, 2Department of Radiology, University of Washington, Seattle, WA, United States, 3Center for Biomedical Imaging Research, School of Medicine, Tsinghua University, Beijing, China
Synopsis
A novel method that exploits spatial-temporal correlation between pixels was developed to segment the dynamic MRA (dMRA) of intracranial arteries. Preliminary results on 3 healthy volunteers demonstrate that the proposed method can effectively identify distal vessels with low signal intensity.
Purpose
Brain dynamic MRA (dMRA) can be used to monitor the blood flow in brain, and has potential to generate multiple quantitative intracranial hemodynamic parametric maps for disease diagnosis [1][2]. Automatic detection and segmentation of vessels in dMRA is important for facilitating the application of dMRA in clinical practice. While vessel segmentation on conventional static MRA, such as TOF-MRA, has been extensively studied previously, few studies have been focused on vessel segmentation on dMRA [3][4][5]. The additional information along the temporal dimension of dMRA data may be very helpful to vessel segmentation, considering that the blood flow and static tissue are expected to have very different temporal signal. In this study, we proposed to segment the intracranial arteries based on dMRA by exploiting the spatial-temporal correlation between vessel voxels.Methods
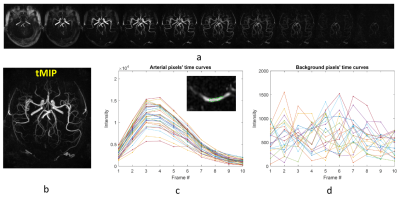
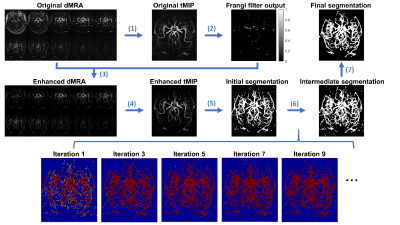
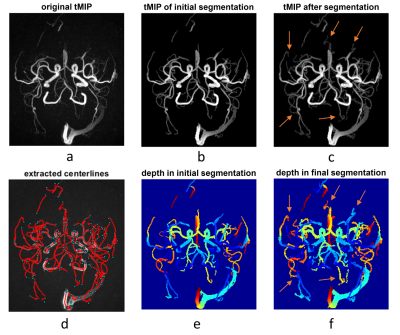
Data: dMRA was acquired with a recently-proposed sequence named iSNAP [6], which generates dMRA by combining arterial spin labeling (ASL) preparation and 3D golden angle radial acquisition and allows measuring multiple intracranial vascular image contrasts in a single scan, on 3 healthy volunteers (25.3 years, 2 males). The dMRA data were generated by performing subtraction between the multiple delay ASL control and label images. The imaging parameters for iSNAP-dMRA were: field of view 204.8×179.2×137.5 mm3, voxel size 0.8 mm isotropic, temporal resolution 197.6 ms, 10 frames. Typical iSNAP dMRA images and the time curves in vessel and background static tissue are shown in Fig.1.Vessel segmentation: The pipeline for the proposed vessel segmentation method is shown in Fig.2. Generation of initial segmentation: A 3D image volume was generated from the 4D MRA by performing a maximum intensity projection along the temporal dimension (tMIP, Fig. 1b). Then, tMIP was processed with Frangi filter [7] to enhance the columnar structure. The output of Frangi filter was multiplied with original 4D MRA, and then another tMIP was generated, which was further processed with thresholding method to generate the initial segmentation, with the threshold value set to the mean plus 2 standard deviation of all pixels’ signal intensity. Iterative vessel segmentation expansion based on spatial-temporal correlation: In each iteration, the pixels to be tested were those in the spherical neighboring region (with a radius of 2 pixels) of the segmented vessel pixels. For each pixel to be tested, a series of correlation coefficient values and the corresponding P values were calculated by performing Pearson correlation between temporal signal of the pixel and all its neighboring, segmented vessel pixels. The tested pixel was determined to be a vessel pixel when the average correlation coefficient value was larger than a threshold value and the average P value was smaller than a threshold value. Such expansion of vessel segmentation was performed iteratively until only a small number (i.e. zero in this study) of new vessel pixels were found. Considering that the signal intensity, thus susceptibility to noise, vary significantly between pixels, especially between the pixels of proximal large arteries and distal small arteries, adaptative threshold values of the correlation coefficients and P values were used by considering the intensity difference between the tested pixel and its neighboring, segmented vessel pixels. Segmentation cleanup: Given that the vessel pixels are expected to be connected with each other, the connected sets with small number of pixels were removed from the final segmentation. Centerline extraction: The skeleton extraction method was applied to the segmentation to extract vessel centerlines, and then spline interpolation and Savitzky-Golay filtering was used for smoothing.
Results and Discussion
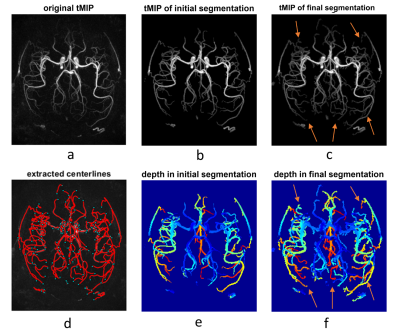
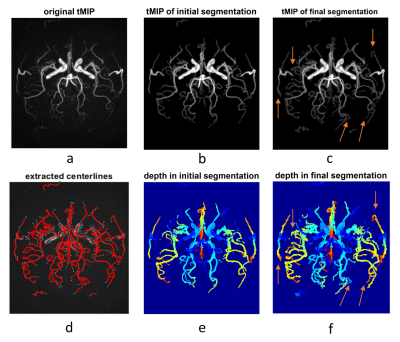
Results of the segmentation and centerline extraction are shown in Fig.3-5 for the 3 subjects, respectively. Around 15 iterations were needed for the iterative segmentation expansion (i.e. Step 6 in Fig. 2). It is obvious that the proposed correlation-based method can extract many more vessels, especially the distal vessels with low signal intensity, than the threshold-based method in the initial segmentation. This suggests the effectiveness of using the spatial-temporal correlation to improve the segmentation. Based on visual inspection, the correlation-based method expanded the segmentation slightly on boundaries of the proximal large vessels, leading to slightly larger vessel size than the initial thresholding-based segmentation. This indicates that the correlation-based could identify the pixels with fractional luminal component, although such pixels have low signal intensity due to partial volume effect. However, such capability of the correlation-based method may lead to more noisy vessel boundaries, which should be further addressed in the future. Overall, the extracted and smoothed vessel centerlines look reasonable, although there exist some unnecessary nodes, which may be due to the noisy boundaries in the final segmentation.In summary, our current results have demonstrated the usefulness of the spatial-temporal correlation between voxels in dMRA in improving segmentation of intracranial vessels. In the future, we will combine such spatial-temporal correlation method with conventional vascular segmentation methods that were developed for non-dynamic MRA, to further improve the segmentation. A solid comparison of the proposed method and the existing methods on a larger dataset will also be performed. By doing these, we expect to achieve automatic brain arterial blood flow quantification and abnormality detection based on iSNAP sequence, which allows simultaneous imaging of intracranial blood flow, luminal geometry and vessel wall [1].
Acknowledgements
No acknowledgement found.References
[1] Schubert T , Wu Y , Johnson K M , et al. Time-of-Arrival Parametric Maps and Virtual Bolus Images Derived From Contrast-Enhanced Time-Resolved Radial Magnetic Resonance Angiography Improve the Display of Brain Arteriovenous Malformation Vascular Anatomy[J]. Investigative Radiology, 2016, 51(11):706.
[2] Shao X , Zhao Z , Russin J , et al. Quantification of intracranial arterial blood flow using noncontrast enhanced 4D dynamic MR angiography[J]. Magnetic Resonance in Medicine, 2019.
[3] Phellan R, Lindner T, Falcão A X, et al. Vessel segmentation in 4D arterial spin labeling magnetic resonance angiography images of the brain[C]//Medical Imaging 2017: Computer-Aided Diagnosis. International Society for Optics and Photonics, 2017, 10134: 101341B.
[4] Phellan R, Linder T, Helle M, et al. Robust cerebrovascular segmentation in 4D ASL MRA images[C]//2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018). IEEE, 2018: 1348-1351.
[5] Phellan R, Lindner T, Helle M, et al. Automatic temporal segmentation of vessels of the brain using 4D ASL MRA images[J]. IEEE transactions on biomedical engineering, 2017, 65(7): 1486-1494.
[6] Chen Z, Zhou Z, Qi H, et al. A novel sequence for simultaneous measurement of whole‐brain static and dynamic MRA, intracranial vessel wall image, and T1‐weighted structural brain MRI[J]. Magnetic Resonance in Medicine, 2020, 85(1): 316-325.
[7] Frangi R F , Niessen W J , Vincken K L , et al. Multiscale vessel enhancement filtering[J]. Lecture Notes in Computer Science, 1998, 1496.
Figures