1628
Quantitative evaluation of signal intensity threshold for carotid plaque components in cardiovascular MR imaging: Validation by histology
Dongye Li1, Huiyu Qiao2, Yongjun Qiao2, Hualu Han2, Dandan Yang2, Jingli Cao3, Huimin Xu4, Tao Wang4, Jun Shen1, and Xihai Zhao2
1Department of Radiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China, 2Department of Biomedical Engineering, Center for Biomedical Imaging Research, Tsinghua University School of Medicine, Beijing, China, 3China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Peking University Third Hospital, Beijing, China
1Department of Radiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China, 2Department of Biomedical Engineering, Center for Biomedical Imaging Research, Tsinghua University School of Medicine, Beijing, China, 3China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Peking University Third Hospital, Beijing, China
Synopsis
It is important to accurately identify carotid necrotic core (NC), intraplaque hemorrhage (IPH) due to its strong association with ischemic stroke. Multi-contrast cardiovascular magnetic resonance (CMR) vessel wall imaging has become an effective non-invasive technique to assess carotid plaque components. The aim of this study sought to confirm criteria of signal intensity threshold of all plaque components by comparing multiple tissue references validated by histology. This study afforded a standard of the signal intensity threshold ratio of carotid plaque components compared to reference tissues on multi-contrast cardiovascular MR imaging, and provides the basis for accurate identification of crucial plaque components.
Introduction and purpose
It has been shown that carotid vulnerable atherosclerotic plaque is the key etiology of ischemic stroke [1]. Histologically, intraplaque hemorrhage (IPH) and large necrotic core (NC) have been considered as the key features of vulnerable plaques [2]. In addition, investigators found that loose matrix (LM) was strongly associated with inflammation of atherosclerosis plaque [3]. Therefore, it is important to accurately characterize vulnerable plaque components. Multi-contrast cardiovascular magnetic resonance (CMR) vessel wall imaging, including time-of-flight (TOF), T1-weighted, T2-weightedand MP-RAGE sequences, has become an effective non-invasive technique to assess carotid plaque components [4,5]. Furthermore, previous serial studies have indicated that dynamic changes of plaque components intensity exist are very important for its effective identification[4]. However, studies to determine the optimum objective criteria for these component detections in Multi-contrast CMR imaging with histology validation are lacking. The aim of this study sought to confirm criteria of intensity threshold of all crucial plaque components by comparing multiple tissue references that afforded the highest diagnostic accuracy using histology as the gold standard.Methods
Study sample: Patients with recent cerebrovascular symptoms carotid atherosclerotic disease (>50%, <70% stenosis and carotid atherosclerotic disease (>70% stenosis) were recruited and underwent carotid MR imaging and CEA. All patients underwent MRI within 1 week before surgery to reduce potential bias in the correlation between images and histology.MR imaging: Carotid MR imaging was performed on a 3.0T MR scanner (Philips, Achieva TX) with 8-channel carotid coil. The MR Imaging protocol included TOF, T1w, T2-weighted (T2w) and MP-RAGE sequences. The imaging parameters are detailed in Figure 1. Histological Sample Processing and Review: After CEA, the samples were sectioned (10μm) every 0.5mm throughout the length of the specimen and stained (hematoxylin and eosin[H&E]). The histological sections were matched with the CMR images by experienced pathologist and radiologist according to the position and the shape of lumen, wall, and plaques with the landmarks of bifurcation. The NC, IPH, LM and lipid pool (LP) were identified and quantified on each matched histological section and MR imaging (Figure2) by two experienced pathologists with>5 years’ experience using ImageJ software and published criteria with consensus [6, 7]. Image review: Two experienced radiologists blind to histology interpreted the MR images matched to histologic specimens with consensus. The boundary of lumen and wall and SMC were outline by using 2D CASCADE and ImageJ software, respectively. The NC, IPH, LM and lipid pool (LP) were identified on MR imaging by using Radiant software. Statistical analysis: The presence or absence of NC, IPH, LM and LP were measured on MR imaging referred to published criteria with consensus [2,4]. The agreement between multi-contrast MR imaging and histology in identifying NC, IPH and LM was determined using Cohen’s Kappa analysis. The signal intensity threshold ratio of NC, IPH, LM and LP referred to SCM and normal artery wall (NAW) were calculated. The study protocol was approved by institutional review board and consent form was obtained from all subjects.Results
In total, 34 subjects (mean age: 63.1 ± 6.3 years, 27 males) with arteries 109 slices having acceptable image quality were included and eligible for MR and histologic image review. Of 109 sections, 69 (63.3%), 45 (41.3%), 59 (54.1%) and 12 (11.0%) sections were found have NC, IPH, LM and LP on the histological sections, respectively. The kappa value of multi-contrast cardiovascular MR imaging agreement with histology in identifying NC, IPH and LM was 0.61, 0.69 and 0.44 (all p<0.001, figure 3), respectively. NC and LP have lower signal intensity threshold ratio compared with IPH and LM on TOF and MP-RAGE imaging referred to reference tissue (Figure 4). IPH and LM have higher signal intensity threshold ratio compared with another plaque components on T1w and T2w imaging (Figure 4). The signal intensity of IPH was higher than other plaque components on T1w, TOF and MP-RAGE imaging (Figure 5).Discussion and Conclusion
In this study, we investigated the signal intensity threshold of all plaque components by comparing multiple tissue references by using histology as the gold standard. And The multi-contrast cardiovascular MR images show moderate agreement with histology in identifying of carotid NC, IPH and LM. This study calculated and afforded a standard of the signal intensity threshold ratio of different carotid plaque components compared with reference tissues on multi-contrast cardiovascular MR imaging. This standard provides basis for the accuracy identification of plaque components by both manual interpretation and automatic segmentation detecting.Acknowledgements
None.References
- Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, Lima JA, Bluemke DA. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the multi-ethnic study of atherosclerosis (MESA). Radiology. 2014;271:381–9.
- Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, Cai J, Ferguson MS, Yuan C. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–7.
- Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006; 241(2):459-68.
- Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–73.
- Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44.
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74.
Figures
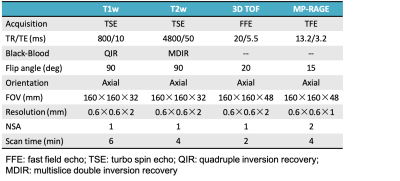
Figure 1. The detail MR sequence parameters
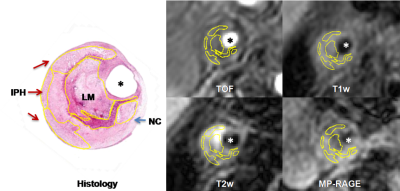
Figure 2. The NC, IPH and LM were identified and quantified on matched histological section and MR imaging
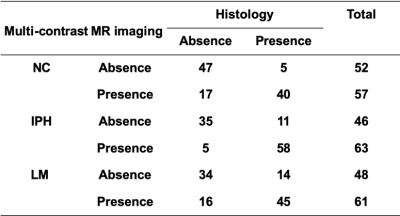
Figure 3. The agreement between multi-contrast MR imaging and histology in identifying NC, IPH and LM
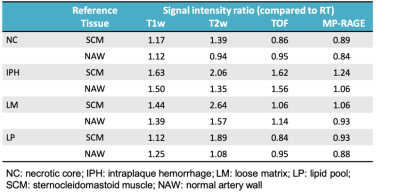
Figure 4. The signal intensity threshold ratio of plaque components referred to reference tissue
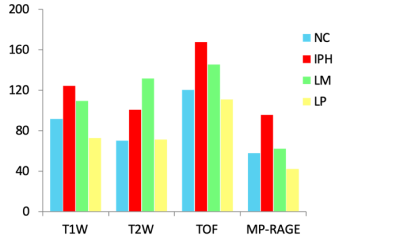
Figure 5. The signal intensity of plaque components in multi-contrast MR imaging