1627
Dual-echo 3D-MERGE with Adiabatic Flow Suppression for Large-coverage Carotid Vessel Wall Imaging1Philips Research North America, Cambridge, MA, United States, 2Vascular Imaging Lab, University of Washington, Seattle, WA, United States, 3Philips Research Hamburg, Hamburg, Germany
Synopsis
In this work, a dual-echo 3D-MERGE with adiabatic flow suppression was developed to improve the image quality of large-coverage carotid vessel wall imaging (VWI). The initial experiments on plaque specimen and healthy volunteers have demonstrated its potential for plaque component analysis and feasibility to achieve isotropic 0.8mm VWI with improved fat/flow suppression and additional quantitative fat fraction/field maps by using this ~4min large-coverage scan.
Introduction
Stroke remains the second leading cause of death worldwide1, and approximately 18%~25% of all strokes are due to carotid atherosclerotic disease2. 3D-MERGE3 has been developed to be one of the fast MR carotid vessel wall imaging (VWI) for plaque burden detection. However, both spectral presaturation inversion recovery (SPIR) based fat suppression and improved Motion Sensitized Driven Equilibrium (iMSDE) based flow suppression are sensitive to B0 and B1 inhomogeneity, which results in deteriorated image quality for large-coverage carotid vessel wall screening. In this work, we aim to improve the image quality of 3D-MERGE scan for large-coverage carotid VWI by using adiabatic iMSDE preparation and dual-echo DIXON approach. The feasibility of this approach has been investigated with plaque specimen and healthy volunteer experiments.Methods
3D-MERGE with Adiabatic iMSDE Preparation and Dual-echo AcquisitionTo reduce the sensitivity to B0 and B1 variations in large-coverage 3D-MERGE scans, adiabatic refocusing pulse was used to replace the composite hard pulse in iMSDE preparation. In addition, dual-echo acquisition and DIXON water fat separation were used to suppress fat for large-coverage carotid VWI, which can offer additional quantitative parametric maps (e.g. fat fraction, field map) for plaque component characterization.
Water Fat Separation using a VARPRO based Projected Power Method
The dual-echo gradient echo signals $$$s_{n}, n\in\{1,2\}$$$ in each voxel were represented by a 7-peak fat spectral model:
$$s_{n} = \left(\rho_{w}+c_{n}\rho_{f}\right)e^{j2\pi\Delta B_{0}\left(n-1\right)\Delta TE} + \epsilon_{n},$$
where $$$\rho_{w}$$$ and $$$\rho_{f}$$$ correspond to water and fat image, $$$c_{n}$$$ represents the dephasing factor at the nth echo time (TE) with pre-calibrated 7-peak fat frequency offsets and amplitudes4, ∆B0 is the field map, ∆TE is the time interval between two consecutive echoes, and $$$\epsilon_{n}$$$ indicates the zero-mean Gaussian noise. With the variable projection (VARPRO) method5, this 3-parameter ($$$\rho_{w}$$$, $$$\rho_{f}$$$, ∆B0) nonlinear least square problem can be reformulated as a function of ∆B0:
$$\min_{\Delta B_{0}}\parallel\left(I-A\left(\Delta B_{0}\right)\left(A\left(\Delta B_{0}\right)^{H}A\left(\Delta B_{0}\right)\right)^{-1}A\left(\Delta B_{0}\right)^{H}\right)\left(\begin{array}{c}s_{1}\\ s_{2}\end{array}\right)\parallel_{2}^{2}, (1)$$
where $$$A\left(\Delta B_{0}\right) = diag\left(\begin{array}{c}1 & e^{j2\pi\Delta B_{0}\Delta TE}\end{array}\right)\left(\begin{array}{c}1 & c_{1} \\1 & c_{2} \end{array}\right)$$$, and $$$I$$$ is the identity matrix. Given the estimated ∆B0, $$$\rho_{w}$$$ and $$$\rho_{f}$$$ can be simply obtained by solving a linear least square problem. Considering the spatial smoothness of field map, the 3D dual-echo images were down-sampled to a lower resolution so that a global search of ∆B0 can be efficiently performed on a 1D grid to minimize Eqn. (1). Two ∆B0 candidates for the smallest two local minimums of Eqn. (1) were stored and the phase ambiguity was resolved by a projected power method6. The resolved ∆B0 were up-sampled to the acquired resolution and used as the initial values to iteratively optimize Eqn. (1) by using a bound-constrained Gauss-Newton algorithm.
MR Scans
All MR scans were performed on a Philips Ingenia 3.0T scanner. For tubed plaque specimen scans (using the animal coil), a dual-echo (TE1/∆TE/TR=6.2/1.0/13ms) 3D-MERGE with adiabatic iMSDE was acquired in isotropic 0.8mm resolution and compared with 2D T1 (TE/TR=12.3/550ms) and T2 (TE/TR=60/4000ms) turbo spin echo (TSE) scans in 0.14x0.14x2mm3 resolution. For volunteer scans, 3D-MERGE datasets were acquired by using a combined 32-channel head and 8-channel carotid coil with FOV (FHxAPxRL) = 250x160x160mm3 and isotropic 0.8mm resolution. Two single-echo 3D-MERGE with SPIR fat suppression were scanned with TE/TR=4.5/10ms and compressed sensing (CS)-SENSE factor=2.2, where one using hard pulse iMSDE took 3:18 and the other using adiabatic iMSDE took 3:26. A dual-echo 3D-MERGE with adiabatic iMSDE was acquired in separate TRs with TE1/∆TE/TR=6.2/1.0/13ms, CS-SENSE factor=4.3, scan time=4:10.
Results
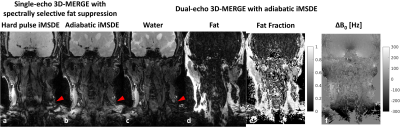
Plaque Specimen ExperimentFigure 1 (c)-(f) show the decomposed water/fat images and fat fraction/field maps from the dual-echo 3D-MERGE scan in this plaque specimen experiment. The T2* weighted water image illustrates hypointense signals in the plaque region, and the non-uniform field map also indicates severe susceptibility variations in this region. These signal characteristics correspond well to the calcification (dark in both T1 and T2 TSE) and hemorrhage (bright in T1 TSE) regions. In addition, both the fat image and fat fraction map imply the presence of lipid, which provides additional information beyond the conventional T1 and T2 weighted plaque imaging.
Volunteer Experiment
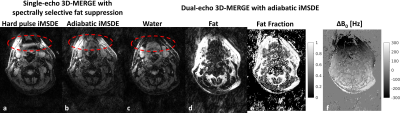
Figure 2 & 3 show the volunteer imaging results with different 3D-MERGE scans. With adiabatic iMSDE preparation, the signal intensity variation caused by the B0 and B1 inhomogeneity can be largely reduced in comparison to hard pulse iMSDE, where some segments of common carotid vessel wall are completely invisible. By using dual-echo DIXON approach, the fat signal can be also better suppressed within this large imaging FOV (red arrows in Fig. 2, red circles in Fig. 3). The dual-echo 3D-MERGE scan can also provide fat fraction and field map for plaque component analysis.
Discussion and Conclusion
Our initial results demonstrate the feasibility of isotropic 0.8mm dual-echo 3D-MERGE with adiabatic iMSDE to improve the fat and flow suppression performance for large-coverage carotid VWI in ~4min. Also, the decomposed fat component and field map worth further investigation for plaque component analysis to distinguish lipid7, calcification8,9, and hemorrhage7-9, which can advance the clinical value of 3D-MERGE beyond plaque burden detection. The proposed water fat separation algorithm can be extended to multi-echo acquisitions for estimation of R2* map and phase/amplitude errors in bipolar gradient scans, which can allow more comprehensive and robust quantitative map estimation for plaque property characterization.Acknowledgements
We would like to thank Dr. Zhang Tao from Subtle Medical for sharing his example code for his dual-echo DIXON using a projected power method.References
1. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417-418.
2. Ooi YC, Gonzalez NR. Management of extracranial carotid artery disease. Cardiol Clin. 2015;33(1):1-35.
3. Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med. 2011;65(3):627-37.
4. Eggers H, Perkins TG, Hussain SM. Influence of Spectral Model and Signal Decay on Hepatic Fat Fraction Measurements at 3T with Dual-Echo Dixon Imaging. ISMRM 2011, p573.
5. Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang ZP. Joint estimation of water/fat images and field inhomogeneity map. Magn Reson Med. 2008;59(3):571-80.
6. Zhang T, Chen Y, Bao S, Alley MT, Pauly JM, Hargreaves BA, Vasanawala SS. Resolving phase ambiguity in dual-echo dixon imaging using a projected power method. Magn Reson Med. 2017;77(5):2066-2076.
7. Koppal S, Warntjes M, Swann J, Dyverfeldt P, Kihlberg J, Moreno R, Magee D, Roberts N, Zachrisson H, Forssell C, Länne T, Treanor D, de Muinck ED. Quantitative fat and R2* mapping in vivo to measure lipid-rich necrotic core and intraplaque hemorrhage in carotid atherosclerosis. Magn Reson Med. 2017;78(1):285-296.
8. Wang C, Zhang Y, Du J, Huszár IN, Liu S, Chen Y, Buch S, Wu F, Liu Y, Jenkinson M, Hsu CC, Fan Z, Haacke EM, Yang Q. Quantitative Susceptibility Mapping for Characterization of Intraplaque Hemorrhage and Calcification in Carotid Atherosclerotic Disease. J Magn Reson Imaging. 2020;52(2):534-541.
9. Nguyen TD, Wen Y, Du J, Liu Z, Gillen K, Spincemaille P, Gupta A, Yang Q, Wang Y. Quantitative susceptibility mapping of carotid plaques using nonlinear total field inversion: Initial experience in patients with significant carotid stenosis. Magn Reson Med. 2020;84(3):1501-1509.
Figures