1620
Peripheral Fresh Blood Imaging with High Spatial Resolution Using Compressed Sensing1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Department of Radiology, Addenbrooke’s Hospital, Cambridge, United Kingdom
Synopsis
A time-efficient high-resolution 3D fresh-blood imaging technique is proposed. The acquisition matrix size was increased from 256 to 512 in both readout and in-plane phase-encoding dimensions. Imaging efficiency was improved using compressed sensing together with k-space subtraction, so that the acquisition time is not prolonged compared to standard-resolution protocols. To avoid flow-related arterial signal voids, velocity-compensation gradients were used instead of velocity-spoiling gradients, and the spoiler gradients in the slice-encoding direction were increased. By decreasing the pixel size, the overall vessel sharpness and depiction of small vessels were significantly improved, at the cost of reducing the contrast-to-noise ratio.
Introduction
Non-contrast-enhanced MR angiography (NCE-MRA) techniques, such as Fresh Blood Imaging (FBI)1,2, are safe and effective tools for the diagnosis of peripheral arterial diseases. In MRA, spatial resolution is critical for the depiction of small vessels and the differentiation between fine grades of stenosis3,4. Peripheral arteries have small branches with diameters less than one millimetre, which places particularly high demands on spatial resolution3. Moreover, peripheral MRA needs a large volume coverage, which requires high imaging efficiency.The resolution in the phase-encoding direction(s) can be improved by acquiring more phase encode steps and applying acceleration techniques to compensate for the increased acquisition times5–7. The resolution in the readout direction can be improved by increasing the readout gradient’s amplitude and/or duration. For FBI, increasing the readout gradients can over-enhance the flow dephasing effect, but this effect can potentially be counteracted by using velocity-compensation gradients1.
In this study, a high-resolution accelerated FBI technique is developed to acquire femoral MRA datasets with increased spatial resolution in the same acquisition time as a current standard-resolution examination. Imaging efficiency was improved by using a compressed-sensing reconstruction method for subtractive MRA, k-space subtraction with phase and intensity correction (KSPIC)8–10. This optimises the sparsity of the k-space subtracted images, allowing large acceleration factors. The resulting images with different resolutions were compared with standard-resolution images using both quantitative metrics and subjective image quality scoring.
Methods
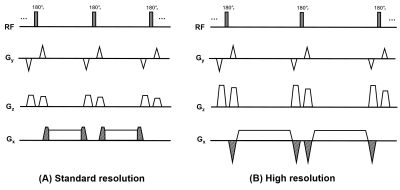
Compared with standard-resolution FBI1,2, three modifications were made to the high-resolution FBI (Figure 1):1. Readout gradients with increased time duration to increase the matrix size.
2. Partial velocity-compensated readout gradients to balance the increased dephasing effect.
3. Increased spoiler gradients along the z-axis (z-spoilers) to suppress the FID signal and its refocused echo explained in Figure 2.
Seven healthy subjects (5 men and 2 women; age range 24–45 years) were imaged using a 1.5 T system (MR450, GE Healthcare, Waukesha, WI). Each subject underwent five coronal 3D femoral artery FBI acquisitions with matrix sizes (x and y dimensions) of 256×256, 320×320, 384×384, 448×448 and 512×512. The corresponding velocity-spoiling/compensation gradient area increases were 20%, −24%, −54%, −74% and −90%, so that the total zeroth moment of the readout gradient remains constant for all resolutions. The area of the z-spoilers was set to 160% for standard-resolution and 240% for high-resolution FBI. (spoiler gradient areas are reported as a percentage of one-half the area of the readout gradient1). The matrix size and resolution in the slice-encoding (z) dimension were 80 and 1.8 mm in all the acquisitions. Other parameters included ETL 58–70, FOV 42 cm, TE 45-60ms, TR 2 or 3 heartbeats.
The total number of k-space samples was 2000 for all acquisitions, corresponding to acceleration factors of 8.0, 10.1, 12.1, 14.1, 16.1 for the acquisitions with matrix sizes from 256 to 512 respectively. The acquisition times were therefore the same over the different resolutions acquired for each subject, circa 3.5–4.5 minutes dependent on the heart rate. The undersampled data were reconstructed using KSPIC. Maximum intensity projections (MIPs) of the images with different resolutions were assessed by two experienced radiologists, in a randomised order for each subject. In addition, contrast-to-noise ratio (CNR) of artery-to-background11 and sharpness assessment12 were used as objective metrics to evaluate the image quality.
Results
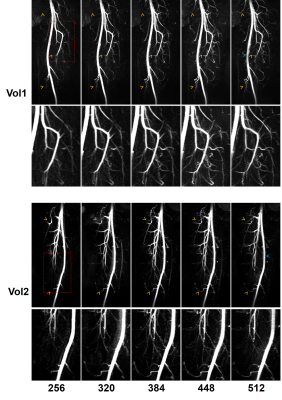
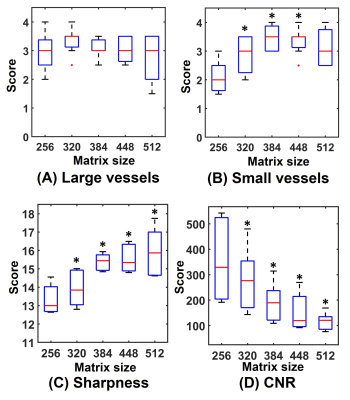
Example MIPs are demonstrated in Figure 3. High-resolution FBI improved the depiction of small arterial branches and sharpness of vessel boundaries. Slight signal loss in the centre of large arteries can be observed on the images with a matrix of 512.Figure 4 shows the subjective and objective evaluation results. For the depiction of large vessels, no significant differences were detected between the images with the standard resolution and those with high resolutions. The depiction of small vessels was significantly improved by increasing the image resolution. Sharpness was significantly improved in all the high-resolution acquisitions, but the CNR of artery-to-background significantly decreased with increasing resolution (P<0.05).
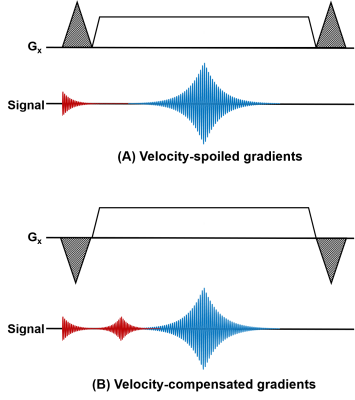
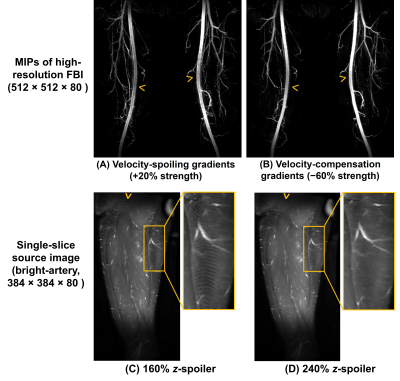
As shown in Figure 5, without the changes to velocity-spoiling/compensation gradients, the increased readout gradient area would lead to an over-enhanced flow dephasing effect, causing arterial signal loss (A). The excessive flow dephasing can be compensated by introducing velocity-compensation gradients (B). Ripple-shaped artefacts can be seen on a high-resolution unsubtracted source image if using the same z-spoiler as standard-resolution FBI (C), but they can be suppressed by increasing the z-spoiler area from 160% to 240% (D).
Discussion and Conclusion
Compared with standard-resolution FBI, high-resolution FBI significantly improved the overall vessel sharpness and depiction of small arterial branches, at the cost of reducing CNR of artery-to-background. No significant difference was observed in the evaluation of the depiction of large arteries between high-resolution and standard-resolution acquisitions. The matrix sizes between 320 and 448 are more appropriate for high-resolution 3D FBI in practice. The improved small-vessel depiction may point toward potential for improving the quantification of tight stenoses, which should be investigated in future patient studies.The resolution in the slice-encode direction was kept the same in all acquisitions and will be evaluated in future work. Future work will assess the technique over the whole leg and acquire more datasets for assessment. Patient studies are also needed to evaluate the diagnostic performance of high-resolution FBI.
Acknowledgements
We thank the NIHR Cambridge Biomedical Research Centre and Addenbrooke’s Charitable Trust for their financial support. Hao Li acknowledges the China Scholarship Council and Cambridge Trust for fellowship support.References
1. Miyazaki M, Takai H, Sugiura S, Wada H, Kuwahara R, Urata J. Peripheral MR angiography: separation of arteries from veins with flow-spoiled gradient pulses in electrocardiography-triggered three-dimensional half-Fourier fast spin-echo imaging. Radiology. 2003;227(3):890-896.
2. Miyazaki M, Sugiura S, Tateishi F, Wada H, Kassai Y, Abe H. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging. 2000;12(5):776-783.
3. Miyazaki M, Lee VS. Nonenhanced MR Angiography. Radiology. 2008;248(1):20-43.
4. Hartung MP, Grist TM, François CJ. Magnetic resonance angiography: current status and future directions. J Cardiovasc Magn Reson. 2011;13(1):19.
5. Çukur T, Lustig M, Nishimura DG. Improving non-contrast-enhanced steady-state free precession angiography with compressed sensing. Magn Reson Med. 2009;61(5):1122-1131.
6. Zenge MO, Vogt FM, Brauck K, et al. High-resolution continuously acquired peripheral MR angiography featuring partial parallel imaging GRAPPA. Magn Reson Med. 2006;56(4):859-865.
7. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195.
8. Li H, Priest AN, Graves MJ, Lomas DJ. Highly Accelerated NCE-MRA Using Complex Subtraction with Intensity Correction: Improved Reconstruction Accuracy and Background Tissue Suppression. In: Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada. ; 2019:2067.
9. Li H, Priest AN, Graves MJ, Lomas DJ. Highly accelerated NCE-MRA: Phase correction to remove background artefacts for complex subtraction. In: Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada. ; 2019:2066.
10. Li H, Graves MJ, Prashar A, Shaida N, Lomas DJ, Priest, AN. Highly Accelerated Subtractive NCE-MRA using Advanced k-space Subtraction and Magnitude Subtraction Reconstruction Methods. In: Proceedings of the 28th Annual Meeting of ISMRM, Paris, France. ; 2020:1324.
11. Akasaka T, Fujimoto K, Yamamoto T, et al. Optimization of regularization parameters in compressed sensing of magnetic resonance angiography: Can statistical image metrics mimic radiologists’ perception? PLoS One. 2016;11(1):1-14.
12. Ahmad R, Ding Y, Simonetti OP. Edge sharpness assessment by parametric modeling: Application to magnetic resonance imaging. Concepts Magn Reson Part A. 2015;44(3):138-149.
Figures