1612
MRI for evaluating compliance of aortic graft in a rat model and comparison to native aorta1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Vascular tissue-engineering provides an alternative solution to replace autologous vessels via developing an artificial vascular graft. To examine the in-vivo fate and understand the clinical potential of the artificial vascular graft, the graft was implanted into the abdominal aorta of Sprague-Dawley rats by end-to-end anastomosis. The goal of this study was to evaluate compliance and vessel wall stiffness in the artic graft and compare results to native aorta using MRI. The results demonstrated that the developed graft has biomechanical characteristics similar to those in native aorta, which may represent a potential avenue to construct a flexible vascular graft in patients.
Introduction
The vascular transplantation is an established therapy for patients who suffer from vascular diseases along with vessel blockage by replacing or bypassing the damaged or obstructed vessels with a functional vascular substitute. Autologous vessels are the first choice as the replacement material for vascular transplant surgery, but the limited tissue source and the high morbidity associated with vessel harvesting have restricted their therapeutic potential. Vascular tissue engineering provides an alternative solution to replace autologous vessels via developing an artificial vascular graft. To examine the in vivo fate and understand the clinical potential of the artificial vascular graft, the graft was implanted into the abdominal aorta of Sprague-Dawley rats by end-to-end anastomosis. The goal of this study was to evaluate compliance and vessel wall stiffness in the aortic graft and compare results to native aorta using non-invasive MRI.Methods
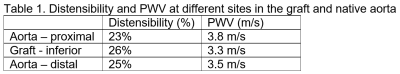
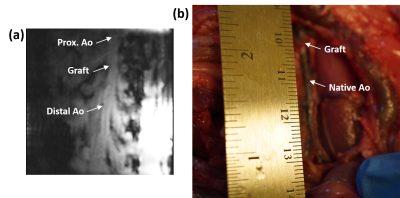
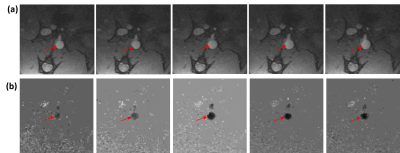
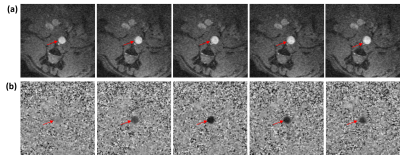
The vascular graft was fabricated with decellularized human amnion membrane with a rolling technique, lyophilization, and chemical crosslinking with 0.5% genipin. Sprague Dawley rats (SD rats, 10-week-old) was anesthetized by isoflurane inhalation. After intravenous injection with 300 IU/kg heparin, the abdominal aorta was clamped in both the proximal and distal regions, and the segment between the clamps was excised. A segment (0.8 cm in length) of graft was connected to the abdominal aorta using an end-to-end anastomosis technique with interrupted stitches at each end (10-0 prolene sutures). Following reperfusion of blood flow through the graft, the incision will be closed in layers. Two rats (one experimental model with 0.8-cm abdominal aortic graft and one normal control) were imaged on a 9.4 T small-animal MRI scanner with 30-cm bore diameter (Bruker, Ettlingen, Germany) using a 4-element surface coil. Rats were scanned in the supine position, with the graft region at the center of the coil. The cardiac MRI exam included scouting images, followed by coronal cine and phase-encoding images at multiple locations above, below, and across the graft in the experimental rat. Coronal time-resolved cine images were acquired in the control rat for comparison. The cine imaging parameters were: TR = 5.1ms, echo time (TE) = 2.1ms, flip angle = 15°, matrix = 176 × 176, field-of-view (FOV) = 40×40 mm2, slice thickness = 1.5mm, acquisition bandwidth = 526 Hz/pixel, number of averages = 3, number of cardiac phases = 30, and scan time ~3 min/slice, depending on heart rate and breathing pattern. The phase-encoding imaging parameters were: TR = 5.5 ms, echo time (TE) = 2.6 ms, flip angle = 15°, matrix = 128 × 128, field-of-view (FOV) = 40×40 mm2, slice thickness = 1.5 mm, velocity-encoding (venc) = 100 cm/s in the slice direction, acquisition bandwidth = 465 Hz/pixel, number of averages = 2, number of cardiac phases = 17, and scan time ~4 min/slice. The images were analyzed to measure distensibility of the graft and of native aorta above (proximal) and below (distal) the graft, as shown in Figure-1, as well as in a control rat. Distensibility was defined as the percentage increase in aortic cross-sectional area between early systole and peak systole. Furthermore, the phase-encoding images were analyzed to measure velocity in the graft and native aorta, from which pulse wave velocity (PWV) was calculated at measurement sites using the flow-area method, as previously described. Briefly, PWV was measured as the slope of the line fitted to the relationship between changes in aortic cross-sectional area and flow during early systole. Statistical t-test was used to test significance in difference between distensibility and PWV in the graft and native aorta. P <0.05 was considered significant.Results
All rats were successfully imaged. The results showed mechanical properties in the graft similar to those in native aorta, as shown in Table-1. Aortic distensibility was 23%, 25%, and 26% in the proximal aorta, distal aorta, and graft, respectively. Pulse wave velocity also showed similar values between graft and native aorta (Figure2). PWV was 3.8 m/s, 3.5 m/s, and 3.3 m/s in the proximal aorta, distal aorta, and graft, respectively. PWV showed inverse relationship with distensibility (correlation = -0.9). In the normal rat, distensibility showed higher average values than those in the model rat (Figure-3). Distensibility was 32%, 30%, and 26% at sites located at positions similar to those imaged in the model rat. However, the difference in aortic distensibility between the model rat and normal rat was not statistically significant (P=0.1).Discussion and Conclusions
The results from this study demonstrated that the graft has biomechanical characteristics similar to those in native aorta and normal rat. The genipin cross-linked vascular graft exhibited ideal integrated layered structure and favorable biocompatibilities in vivo. The main criteria for such a vascular graft include suitable structure, high compliance, low thrombogenicity, and long-term patency. Findings from this research project might generate an in-depth understanding of the cellular and functional mechanisms underlying vascular reendothelialization and regeneration. In conclusion, this MRI-based study showed an approach that may represent a potential avenue to construct a flexible vascular graft that matches the patient-specific dimension, as well as meeting specific demands for biological and mechanical performance criteria through rolling the decellularized amnion membrane with lyophilization and the genipin crosslinking treatment.Acknowledgements
No acknowledgement found.References
1. Cunnane et al, ACS Appl Mater Interfaces12:26955-26965.
2. Krawiec et al. J Vasc Surg 66:883-890.
3. Ibrahim et al, JCMR 12:26.
4. Ibrahim et al, MRI 29:966-974.
Figures