1597
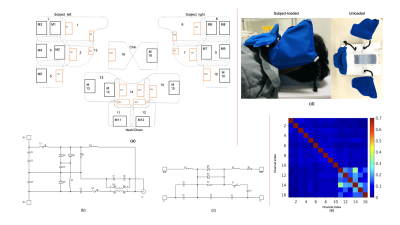
A novel 16 channel flexible coil for highly accelerated upper-airway MRI1Roy J Carver Department of Biomedical Engineering, University of Iowa, iowa city, IA, United States, 2ScanMed LLC, Omaha, NE, United States, 3Department of Radiology, University of Iowa, iowa city, IA, United States, 4Department of Neurology, University of Iowa, iowa city, IA, United States, 5Department of Otolaryngology, University of Iowa, iowa city, IA, United States
Synopsis
We develop a novel custom airway coil that offers significant boost in signal sensitivity in several regions of the upper-airway such as tongue, soft-palate, pharynx, glottis. Our coil is designed to be flexible for easy conformation to the subjects face/neck anatomy. With the proposed coil, we demonstrate robust parallel MRI performance up to R=4-5 fold for static imaging with 1-D under-sampling, and highly accelerated dynamic imaging (up to R~27 fold) of swallowing, and airway shaping due to variations in breathing.
PURPOSE:
The human upper-airway consists of several structures such as the lips, tongue, hard, and soft palate, larynx (including vocal folds), and pharynx – all of which coordinate with great dexterity to perform essential day to day functions of speaking, swallowing, and breathing[1]–[3]. Dynamic MRI has shown great potential to assess the kinematics of these structures in many applications including assessing speech/swallowing disorders, singing, and sleep apnea. However, many previous studies relied on coils that are designed for other parts of the body [3]–[8]. Recently, two sites demonstrated the value of custom coils for accelerated airway MRI [9]–[11]. Here, we built a novel 16 channel flexible anatomy conforming dedicated airway coil at 3 Tesla which provides high sensitivity in all upper-airway regions of interest. We demonstrate its utility in enabling highly accelerated dynamic imaging in the applications of swallowing, and airway shaping due to variations in breathing.METHODS:
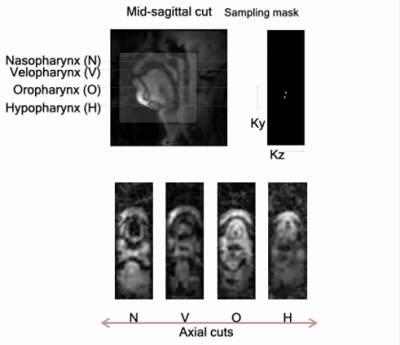
Fig. 1 shows the coil design, which has a left cheek piece (5 elements), a right cheek piece (5 elements), and a neck/chin piece (5+1 elements). These are mounted to a flexible substrate shaped to conform to the subject's face/neck anatomy easily. We imaged three volunteers on a 3T GE Premier scanner with the 16 channel airway coil, 48 channel head coil, and a 21 channel head-neck coil. We acquired fully sampled 3D GRE datasets in the resting posture (flip angle: 5 degrees, Phase x frequency x slice partitions = 128 x 128 x 32 ; FOV = 24 cm x 24 cm x 6.4 cm; scan time = 20 secs). To quantitatively assess parallel MRI performance as a function of reduction factor (R), we performed retrospective 1D uniform under-sampling of the 3D raw data in the superior-inferior directions. We reconstructed the mid-sagittal plane using SENSE with no regularization. Coil maps were derived from central 5% fully sampled k-space lines using a sum of squares approach. We next performed a prospectively accelerated 3D imaging experiment for airway shaping while the subjects were performing Mueller’s maneuver. This maneuver involved forced inspiration with the mouth and nose closed, resulting in airway collapse in the awake state [12]. Cartesian under-sampling in the ky-kz plane along a variable density golden angle spiral view order was implemented [13] (FOV: 24cm x 24cm x 8cm; at 2mm isotropic resolution; flip angle: 5 degrees; readout in the superior/inferior direction). We sorted the data into a dynamic time series with a time resolution of ~500 ms, which corresponded to a net R of ~25 fold. Reconstructed was done via sparse SENSE regularization scheme with spatial and temporal finite difference constraints. We also performed a swallowing experiment, which involved the subjects swallowing ~10 ml bolus of pineapple juice, which served as a natural contrast agent due to inherent manganese content. We implemented a 2D multi-slice variable density spiral GRE sequence with golden angle step interleaving (TR: 5.7 ms; readout duration: 1.2 ms; slice thickness: 6mm; 3 slices: 1 mid-sagittal, and 2 para-sagittal slices). The raw data was sorted at 1 spiral arm/frame, which corresponded to R~27 fold, and a net time resolution of 17.1 ms/frame. Similar to above, reconstruction was performed using sparse SENSE with temporal finite difference regularization in the BART computing environment [14].RESULTS:
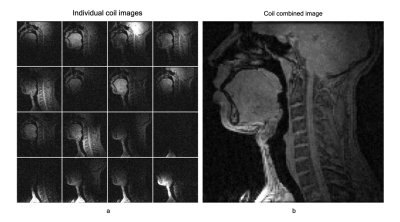
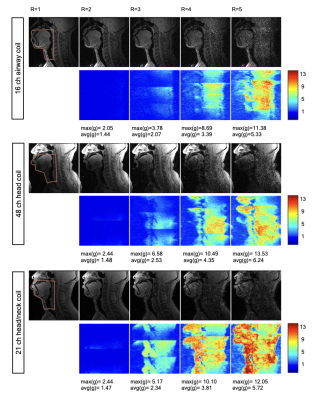
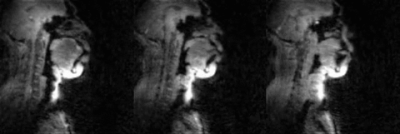
Fig. 2 shows the individual 16 coil images from the airway coil at R=1 and the SENSE coil combined image for a representative sagittal cross-section. This depicts high sensitivity in all upper-airway regions. Fig. 3 shows the 1D SENSE reconstructions and associated g-maps at various Rs. Compared to the head only and head-neck coils, the proposed airway coil shows only a moderate increase in g-factors as R increases and graceful degradation in image quality. Fig. 4 shows an animation of the deforming 3D airway during Mueller’s maneuver, and we observe the kinematics of airway shaping to be well depicted with minimal motion blurring. Finally, Fig. 5 shows an animation of the swallowing bolus's movement in the 3 sagittal planes to be captured with good spatio-temporal fidelity.CONCLUSIONS:
We demonstrated the custom coil to boost signal sensitivity in several regions of the upper-airway significantly. Our results from three volunteers showed robust parallel MRI performance up to R=4-5 fold for static imaging with 1-D under-sampling. The feasibility of high accelerations up to R~27 fold for dynamic 2D multi-slice, and native 3D experiments to capture rapidly varying dynamics during swallowing, and Mueller’s maneuver.Acknowledgements
This work was conducted on an MRI instrument funded by NIH-S10 instrumentation grant: 1S10OD025025-01. We also thank the Roy J Carver Department of Biomedical Engineering, Department of Radiology, and Department of Otolaryngology, the University of Iowa, for funding to support the costs of the airway coil.References
[1] S. R. Shott, “Sleep cine magnetic resonance imaging-A dynamic evaluation of the airway,” Oper. Tech. Otolaryngol. - Head Neck Surg., 2012.
[2] Y. Zu et al., “Evaluation of swallow function after tongue cancer treatment using real-time magnetic resonance imaging: a pilot study.,” JAMA Otolaryngol. Head Neck Surg., vol. 139, pp. 1312–9, 2013.
[3] S. G. Lingala, B. P. Sutton, M. E. Miquel, and K. S. Nayak, “Recommendations for real-time speech MRI,” Journal of Magnetic Resonance Imaging, vol. 43, no. 1. pp. 28–44, 2016.
[4] M. Burdumy et al., “Acceleration of MRI of the vocal tract provides additional insight into articulator modifications,” J. Magn. Reson. Imaging, vol. 42, no. 4, pp. 925–935, 2015.
[5] M. Fu et al., “High-resolution dynamic speech imaging with joint low-rank and sparsity constraints,” Magn. Reson. Med., 2014.
[6] B. P. Sutton, C. Conway, Y. Bae, C. Brinegar, Z.-P. Liang, and D. P. Kuehn, “Dynamic imaging of speech and swallowing with MRI,” in Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE, 2009, pp. 6651–6654.
[7] J. E. Barrera, “Sleep magnetic resonance imaging: Dynamic characteristics of the airway during sleep in obstructive sleep apnea syndrome,” Laryngoscope, vol. 121, no. 6, pp. 1327–1335, 2011.
[8] C. Darquenne et al., “Upper airway dynamic imaging during tidal breathing in awake and asleep subjects with obstructive sleep apnea and healthy controls,” Physiol. Rep., vol. 6, no. 10, pp. 1–9, 2018.[9] S. G. Lingala, Y. Zhu, Y. Kim, A. Toutios, S. Narayanan, and K. S. Nayak, “A fast and flexible MRI system for the study of dynamic vocal tract shaping,” Magn. Reson. Med., vol. 00, p. n/a-n/a, 2016.
[10] Y.-C. Kim, C. E. Hayes, S. S. Narayanan, and K. S. Nayak, “Novel 16-channel receive coil array for accelerated upper airway MRI at 3 Tesla,” Magn. Reson. Med., vol. 65, no. 6, pp. 1711–1717, 2011.
[11] L. Voskuilen et al., “A 12-channel flexible receiver coil for accelerated tongue imaging,” Magn. Reson. Mater. Physics, Biol. Med., 2020.
[12] B. A. Stuck and J. T. Maurer, “Airway evaluation in obstructive sleep apnea,” Sleep Medicine Reviews. 2008.
[13] J. Y. Cheng et al., “Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease,” J. Magn. Reson. Imaging, 2016.
[14] M. Uecker, J. I. Tamir, F. Ong, and M. Lustig, “The BART Toolbox for Computational Magnetic Resonance Imaging,” Ismrm, 2016.
Figures